The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR), and independently by Heinrich Hock in 1944.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.

An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivatives which are known.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.

Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.

Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone. That parent is sometimes simply called quinone, and this is the only hydroxy derivative of it.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
2,5-Dihydroxy-1,4-benzoquinone or 2,5-dihydroxy-para-benzoquinone is an organic compound with formula C
6H
4O
4, formally derived from 1,4-benzoquinone by replacing two hydrogen atoms with hydroxyl (OH) groups. It is one of seven dihydroxybenzoquinone isomers. It is a yellow solid with planar molecules that exhibits ferroelectric properties.

Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.

Tetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula C
10O
10. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid.

In chemistry, 1,4-benzoquinonetetracarboxylic acid is an organic compound with formula C
10H
4O
10, or (C6O2)(-(CO)OH)4, which can be viewed as deriving from para-benzoquinoneC
6H
4O
2 through replacement of the four hydrogen atoms by carboxyl functional groups -(CO)OH.
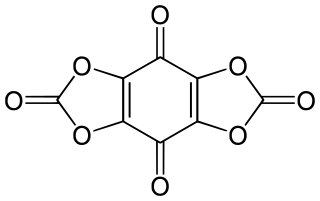
Tetrahydroxy-1,4-benzoquinone biscarbonate is a chemical compound, an oxide of carbon with formula C
8O
8. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two carbonate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and carbonic acid.
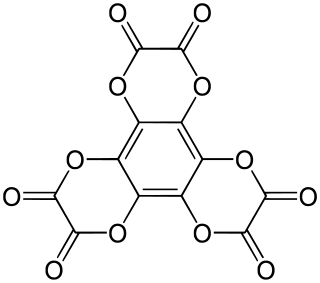
Hexahydroxybenzene trisoxalate is a chemical compound, an oxide of carbon with formula C
12O
12. Its molecule consists of a benzene core with the six hydrogen atoms replaced by three oxalate groups. It can be seen as a sixfold ester of benzenehexol and oxalic acid.

Hexahydroxybenzene triscarbonate is a chemical compound, an oxide of carbon with formula C
9O
9. Its molecular structure consists of a benzene core with the six hydrogen atoms replaced by three carbonate groups. It can be seen as a sixfold ester of hexahydroxybenzene (benzenehexol) and carbonic acid.

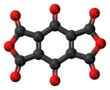
1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.