
A carbonate is a salt of carbonic acid, H2CO3, characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate groupO=C(−O−)2.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

In organic chemistry, a carbonate ester is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R−O−C(=O)−O−R' and they are related to esters, ethers and also to the inorganic carbonates.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is an acid-labile protecting group used in organic synthesis.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.
In chemistry, hyponitrite may refer to the anion N
2O2−
2 ([ON=NO]2−), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R1−O−N=N−O−R2, where R1 and R2 are organic groups. Such compounds can be viewed as salts and esters of hyponitrous acid. An acid hyponitrite is an ionic compound with the anion HN
2O−
2 ([HON=NO]−).

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.
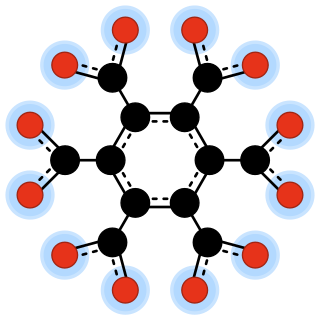
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula C
xOn−
y for some integers x, y, and n.

A dicarbonate, also known as a pyrocarbonate, is a chemical containing the divalent −O−C(=O)−O−C(=O)−O− or −C2O5− functional group, which consists of two carbonate groups sharing an oxygen atom. It is one of polycarbonate functional groups. These compounds can be viewed as derivatives of the hypothetical compound dicarbonic acid, HO−C(=O)−O−C(=O)−OH or H2C2O5. Three important organic compounds containing this group are:
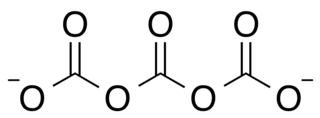
In organic chemistry, a tricarbonate is a compound containing the divalent −O−C(=O)−O−C(=O)−O−C(=O)−O− functional group, which consists of three carbonate groups linked in a chain by sharing of oxygen atoms. These compounds can be viewed as derivatives of a hypothetical tricarbonic acid, HO−C(=O)−O−C(=O)−O−C(=O)−OH. An important example is di-tert-butyl tricarbonate(H3C−)3C−C3O7−C(−CH3)3, an intermediate in the synthesis of di-tert-butyl dicarbonate.




























