
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group, chemically similar to its group neighbours silicon and tin. Pure germanium is a semiconductor with an appearance similar to elemental silicon. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.

Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4).
Germanium tetrachloride is a colourless, fuming liquid with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
Germanium dioxide, also called germanium oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.

Organogermanium compounds are organometallic compounds containing a carbon to germanium or hydrogen to germanium chemical bond. Organogermanium chemistry is the corresponding chemical science. Germanium shares group 14 in the periodic table with silicon, tin and lead, and not surprisingly the chemistry of organogermanium is in between that of organosilicon compounds and organotin compounds.

Gallium trichloride is the chemical compound with the formula GaCl3. Solid gallium trichloride exists as a dimer with the formula Ga2Cl6. It is colourless and soluble in virtually all solvents, even alkanes, which is truly unusual for a metal halide. It is the main precursor to most derivatives of gallium and a reagent in organic synthesis.
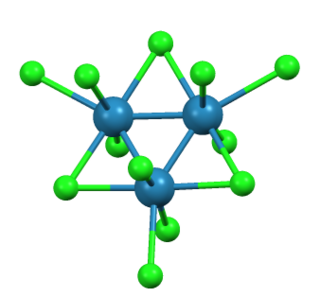
Trirhenium nonachloride is a compound with the formula ReCl3, sometimes also written Re3Cl9. It is a dark red hygroscopic solid that is insoluble in ordinary solvents. The compound is important in the history of inorganic chemistry as an early example of a cluster compound with metal-metal bonds. It is used as a starting material for synthesis of other rhenium complexes.

Trimethyltin chloride is an organotin compound with the formula (CH3)3SnCl. It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.

Germanium monoxide, GeO, is a chemical compound of germanium and oxygen. It can be prepared as a yellow sublimate at 1000 °C by reacting GeO2 with Ge metal. The yellow sublimate turns brown on heating at 650 °C. GeO is not well characterised. It is amphoteric dissolving in acids to form germanium(II) salts and in alkali to form "trihydroxogermanates" or "germanites" containing the Ge(OH)3− ion.
Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a solid and contains germanium in the +2 oxidation state.

Germanium selenide is a chemical compound with the formula GeSe. It exists as black crystalline powder having orthorhombic crystal symmetry; at temperatures ~650 °C, it transforms into the cubic NaCl structure.
Tetrachloride may refer to:

Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.

Lead tetrachloride, also known as lead(IV) chloride, has the molecular formula PbCl4. It is a yellow, oily liquid which is stable below 0 °C, and decomposes at 50 °C. It has a tetrahedral configuration, with lead as the central atom. The Pb–Cl covalent bonds have been measured to be 247 pm and the bond energy is 243 kJ⋅mol−1.
Chalcogenide chemical vapour deposition is a proposed technology for depositing thin films of chalcogenides, i.e. materials derived from sulfides, selenides, and tellurides. Conventional CVD can be used to deposit films of most metals, many non-metallic elements as well as a large number of compounds including carbides, nitrides, oxides. CVD can be used to synthesize chalcogenide glasses.
Germanium(II) hydroxide, normally written as Ge(OH)2 is a poorly characterised compound sometimes called hydrous germanium(II) oxide or germanous hydroxide. It was first reported by Winkler in 1886.
Germanium(II) hydrides, also called germylene hydrides, are a class of Group 14 compounds consisting of low-valent germanium and a terminal hydride. They are also typically stabilized by an electron donor-acceptor interaction between the germanium atom and a large, bulky ligand.
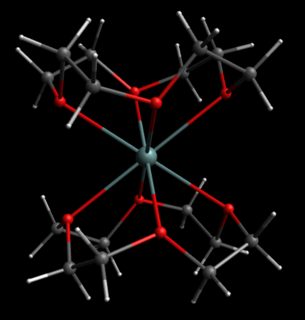
Ge(II) dicationic complexes refer to coordination compounds of germanium with a +2 formal oxidation state, and a +2 charge on the overall complex. In some of these coordination complexes, the coordination is strongly ionic, localizing a +2 charge on Ge, while in others the bonding is more covalent, delocalizing the cationic charge away from Ge. Examples of dicationic Ge(II) complexes are much rarer than monocationic Ge(II) complexes, often requiring the use of bulky ligands to shield the germanium center. Dicationic complexes of Ge(II) have been isolated with bulky isocyanide and carbene ligands. Much more weakly coordinated Germanium (II) dications have been isolated as complexes with polyether ligands, such as crown ethers and [2.2.2]cryptand. Crown ethers and cryptands are typically known for their ability to bind metal cations, however these ligands have also been employed in stabilizing low-valent cations of heavier p-block elements.. A Ge2+ ion's valence shell consists of a filled valence s-orbital but empty valence p-orbitals, giving rise to atypical bonding in these complexes. Germanium is a metalloid of the carbon group, typically forming compounds with mainly covalent bonding, contrasting with the dative bonding observed in these coordination complexes.












