Germanium sulfide may refer to:
- Germanium(IV) sulfide GeS2, also known as Germanium disulfide
- Germanium(II) sulfide GeS, a semiconductor also known as Germanium monosulfide
Germanium sulfide may refer to:

Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
Briartite is an opaque iron-grey metallic sulfide mineral, Cu2(Zn,Fe)GeS4 with traces of Ga and Sn, found as inclusions in other germanium-gallium-bearing sulfides.

Clemens Alexander Winkler was a German chemist who discovered the element germanium in 1886, solidifying Dmitri Mendeleev's theory of periodicity.

Argyrodite is an uncommon silver germanium sulfide mineral with formula Ag8GeS6. The color is iron-black with a purplish tinge, and the luster metallic.

Germanium tetrachloride is a colourless, fuming liquid with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.
Ge or GE may refer to:
GES or Gęś may refer to:
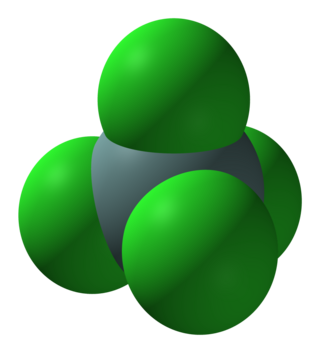
Germanium chloride may refer to:
Germanium oxide may refer to:
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.
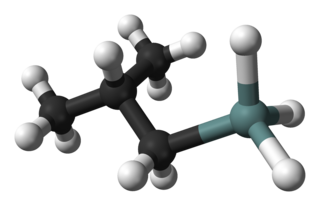
Isobutylgermane (IBGe, Chemical formula: (CH3)2CHCH2GeH3, is an organogermanium compound. It is a colourless, volatile liquid that is used in MOVPE (Metalorganic Vapor Phase Epitaxy) as an alternative to germane. IBGe is used in the deposition of Ge films and Ge-containing thin semiconductor films such as SiGe in strained silicon application, and GeSbTe in NAND Flash applications.
In solid-state physics, a metal–semiconductor (M–S) junction is a type of electrical junction in which a metal comes in close contact with a semiconductor material. It is the oldest practical semiconductor device. M–S junctions can either be rectifying or non-rectifying. The rectifying metal–semiconductor junction forms a Schottky barrier, making a device known as a Schottky diode, while the non-rectifying junction is called an ohmic contact.

Germanium disulfide or Germanium(IV) sulfide is the inorganic compound with the formula GeS2. It is a white high-melting crystalline solid. The compound is a 3-dimensional polymer, in contrast to silicon disulfide, which is a one-dimensional polymer. The Ge-S distance is 2.19 Å.
Tetrachloride may refer to:
Germanium fluoride is a chemical compound of germanium and fluorine which exists in the following forms:

Germanium monosulfide or Germanium(II) sulfide is the chemical compound with the formula GeS. It is a chalcogenide glass and a semiconductor. Germanium sulfide is described as a red-brown powder or black crystals. Germanium(II) sulfide when dry is stable in air, hydrolyzes slowly in moist air but rapidly reacts in water forming Ge(OH)2 and then GeO. It is one of a few sulfides that can be sublimed under vacuum without decomposition.
Sulfidogermanates or thiogermanates are chemical compounds containing anions with sulfur atoms bound to germanium. They are in the class of chalcogenidotetrelates. Related compounds include thiosilicates, thiostannates, selenidogermanates, telluridogermanates and selenidostannates.
Germanium compounds are chemical compounds formed by the element germanium (Ge). Germanium is insoluble in dilute acids and alkalis but dissolves slowly in hot concentrated sulfuric and nitric acids and reacts violently with molten alkalis to produce germanates ([GeO
3]2−
). Germanium occurs mostly in the oxidation state +4 although many +2 compounds are known. Other oxidation states are rare: +3 is found in compounds such as Ge2Cl6, and +3 and +1 are found on the surface of oxides, or negative oxidation states in germanides, such as −4 in Mg
2Ge. Germanium cluster anions (Zintl ions) such as Ge42−, Ge94−, Ge92−, [(Ge9)2]6− have been prepared by the extraction from alloys containing alkali metals and germanium in liquid ammonia in the presence of ethylenediamine or a cryptand. The oxidation states of the element in these ions are not integers—similar to the ozonides O3−.