Related Research Articles

The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.

Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products ; this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes the following applications:

Vapour pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.

Coke is a grey, hard, and porous coal-based fuel with a high carbon content and few impurities, made by heating coal or oil in the absence of air—a destructive distillation process. It is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges when air pollution is a concern.

Bituminous coal, or black coal, is a type of coal containing a tar-like substance called bitumen or asphalt. Its coloration can be black or sometimes dark brown; often there are well-defined bands of bright and dull material within the seams. It is typically hard but friable. Its quality is ranked higher than lignite and sub-bituminous coal, but lesser than anthracite. It is the most abundant rank of coal, with deposits found around the world, often in rocks of Carboniferous age. Bituminous coal is formed from sub-bituminous coal that is buried deeply enough to be heated to 85 °C (185 °F) or higher.
In chemical thermodynamics, activity is a measure of the "effective concentration" of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution. The term "activity" in this sense was coined by the American chemist Gilbert N. Lewis in 1907.
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulated by the English chemist William Henry, who studied the topic in the early 19th century.

In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large hydrocarbons into smaller, more useful alkanes and alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures.

Solid fuel refers to various forms of solid material that can be burnt to release energy, providing heat and light through the process of combustion. Solid fuels can be contrasted with liquid fuels and gaseous fuels. Common examples of solid fuels include wood, charcoal, peat, coal, hexamine fuel tablets, dry dung, wood pellets, corn, wheat, rye, and other grains. Solid fuels are extensively used in rocketry as solid propellants. Solid fuels have been used throughout human history to create fire and solid fuel is still in widespread use throughout the world in the present day.
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition.

Petroleum products are materials derived from crude oil (petroleum) as it is processed in oil refineries. Unlike petrochemicals, which are a collection of well-defined usually pure organic compounds, petroleum products are complex mixtures. Most petroleum is converted into petroleum products, which include several classes of fuels.
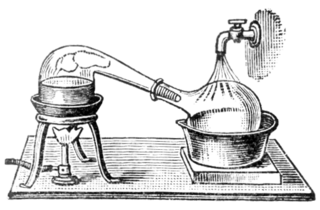
Destructive distillation is a chemical process in which decomposition of unprocessed material is achieved by heating it to a high temperature; the term generally applies to processing of organic material in the absence of air or in the presence of limited amounts of oxygen or other reagents, catalysts, or solvents, such as steam or phenols. It is an application of pyrolysis. The process breaks up or 'cracks' large molecules. Coke, coal gas, gaseous carbon, coal tar, ammonia liquor, and coal oil are examples of commercial products historically produced by the destructive distillation of coal.

Petroleum coke, abbreviated coke or petcoke, is a final carbon-rich solid material that derives from oil refining, and is one type of the group of fuels referred to as cokes. Petcoke is the coke that, in particular, derives from a final cracking process—a thermo-based chemical engineering process that splits long chain hydrocarbons of petroleum into shorter chains—that takes place in units termed coker units. Stated succinctly, coke is the "carbonization product of high-boiling hydrocarbon fractions obtained in petroleum processing ". Petcoke is also produced in the production of synthetic crude oil (syncrude) from bitumen extracted from Canada’s tar sands and from Venezuela's Orinoco oil sands.
Coal analysis techniques are specific analytical methods designed to measure the particular physical and chemical properties of coals. These methods are used primarily to determine the suitability of coal for coking, power generation or for iron ore smelting in the manufacture of steel.

Fragrance extraction refers to the separation process of aromatic compounds from raw materials, using methods such as distillation, solvent extraction, expression, sieving, or enfleurage. The results of the extracts are either essential oils, absolutes, concretes, or butters, depending on the amount of waxes in the extracted product.

The Fenske equation in continuous fractional distillation is an equation used for calculating the minimum number of theoretical plates required for the separation of a binary feed stream by a fractionation column that is being operated at total reflux.
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.
Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. By convention, relative volatility is usually denoted as .

In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law.

Visakhapatnam Steel Plant is the integrated steel plants of Rashtriya Ispat Nigam Limited in Visakhapatnam. Founded in 1982, the plant focuses on producing value-added steel, producing 5.773 million tonnes of hot metal, 5.272 million tonnes of crude steel and 5.138 million tonnes of saleable steel in the 2021-2022 financial year. According to the India Daily Times, the plant is expected to skyrocket in terms of production in the nearest future.
References
- IUPAC Compendium of Chemical Terminology 2nd Edition (1997)