Morphogenesis is the biological process that causes a cell, tissue or organism to develop its shape. It is one of three fundamental aspects of developmental biology along with the control of tissue growth and patterning of cellular differentiation.

Metastasis is a pathogenic agent's spreading from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, are metastases (mets). It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues.

Catenins are a family of proteins found in complexes with cadherin cell adhesion molecules of animal cells. The first two catenins that were identified became known as α-catenin and β-catenin. α-Catenin can bind to β-catenin and can also bind filamentous actin (F-actin). β-Catenin binds directly to the cytoplasmic tail of classical cadherins. Additional catenins such as γ-catenin and δ-catenin have been identified. The name "catenin" was originally selected because it was suspected that catenins might link cadherins to the cytoskeleton.
The epithelial–mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal cells that can differentiate into a variety of cell types. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation. EMT has also been shown to occur in wound healing, in organ fibrosis and in the initiation of metastasis in cancer progression.
Intravasation is the invasion of cancer cells through the basement membrane into a blood or lymphatic vessel. Intravasation is one of several carcinogenic events that initiate the escape of cancerous cells from their primary sites. Other mechanisms include invasion through basement membranes, extravasation, and colonization of distant metastatic sites. Cancer cell chemotaxis also relies on this migratory behavior to arrive at a secondary destination designated for cancer cell colonization.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.
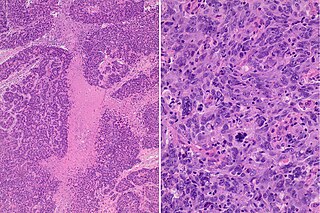
The basal-like carcinoma is a recently proposed subtype of breast cancer defined by its gene expression and protein expression profile.

Epithelial cell adhesion molecule (EpCAM), also known as CD326 among other names, is a transmembrane glycoprotein mediating Ca2+-independent homotypic cell–cell adhesion in epithelia. EpCAM is also involved in cell signaling, migration, proliferation, and differentiation. Additionally, EpCAM has oncogenic potential via its capacity to upregulate c-myc, e-fabp, and cyclins A & E. Since EpCAM is expressed exclusively in epithelia and epithelial-derived neoplasms, EpCAM can be used as diagnostic marker for various cancers. It appears to play a role in tumorigenesis and metastasis of carcinomas, so it can also act as a potential prognostic marker and as a potential target for immunotherapeutic strategies.
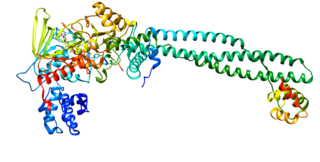
Zinc finger protein SNAI1 is a protein that in humans is encoded by the SNAI1 gene. Snail is a family of transcription factors that promote the repression of the adhesion molecule E-cadherin to regulate epithelial to mesenchymal transition (EMT) during embryonic development.

SCRIB, also known as Scribble, SCRIBL, or Scribbled homolog (Drosophila), is a scaffold protein which in humans is encoded by the SCRIB gene. It was originally isolated in Drosophila melanogaster in a pathway (also known as the Scribble complex) with DLGAP5 (Discs large) and LLGL1 (Lethal giant larvae) as a tumor suppressor. In humans, SCRIB is found as a membrane protein and is involved in cell migration, cell polarity, and cell proliferation in epithelial cells. There is also strong evidence that SCRIB may play a role in cancer progression because of its strong homology to the Drosophila protein.

Cadherin-1 or Epithelial cadherin(E-cadherin), is a protein that in humans is encoded by the CDH1 gene. Mutations are correlated with gastric, breast, colorectal, thyroid, and ovarian cancers. CDH1 has also been designated as CD324. It is a tumor suppressor gene.
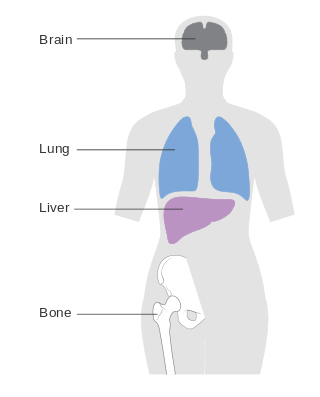
Metastatic breast cancer, also referred to as metastases, advanced breast cancer, secondary tumors, secondaries or stage IV breast cancer, is a stage of breast cancer where the breast cancer cells have spread to distant sites beyond the axillary lymph nodes. There is no cure for metastatic breast cancer; there is no stage after IV.
A mesenchymal–epithelial transition (MET) is a reversible biological process that involves the transition from motile, multipolar or spindle-shaped mesenchymal cells to planar arrays of polarized cells called epithelia. MET is the reverse process of epithelial–mesenchymal transition (EMT) and it has been shown to occur in normal development, induced pluripotent stem cell reprogramming, cancer metastasis and wound healing.
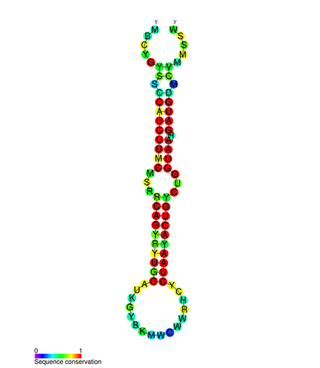
In molecular biology, the miR-200 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by binding and cleaving mRNAs or inhibiting translation. The miR-200 family contains miR-200a, miR-200b, miR-200c, miR-141, and miR-429. There is growing evidence to suggest that miR-200 microRNAs are involved in cancer metastasis.

The hallmarks of cancer were originally six biological capabilities acquired during the multistep development of human tumors and have since been increased to eight capabilities and two enabling capabilities. The idea was coined by Douglas Hanahan and Robert Weinberg in their paper "The Hallmarks of Cancer" published January 2000 in Cell.

Vasculogenic mimicry (VM) is a strategy used by tumors to ensure sufficient blood supply is brought to its cells through establishing new tumor vascularization. This process is similar to tumor angiogenesis; on the other hand vascular mimicry is unique in that this process occurs independent of endothelial cells. Vasculature is instead developed de novo by cancer cells, which under stress conditions such as hypoxia, express similar properties to stem cells, capable of differentiating to mimic the function of endothelial cells and form vasculature-like structures. The ability of tumors to develop and harness nearby vasculature is considered one of the hallmarks of cancer disease development and is thought to be closely linked to tumor invasion and metastasis. Vascular mimicry has been observed predominantly in aggressive and metastatic cancers and has been associated with negative tumor characteristics such as increased metastasis, increased tissue invasion, and overall poor outcomes for patient survival. Vascular mimicry poses a serious problem for current therapeutic strategies due to its ability to function in the presence of Anti-angiogenic therapeutic agents. In fact, such therapeutics have been found to actually drive VM formation in tumors, causing more aggressive and difficult to treat tumors to develop.
Breast cancer is the most prevalent type of cancer among women globally, with 685,000 deaths recorded worldwide in 2020. The most commonly used treatment methods for breast cancer include surgery, radiotherapy and chemotherapy. Some of these treated patients experience disease relapse and metastasis. The aggressive progression and recurrence of this disease has been attributed the presence of a subset of tumor cells known as breast cancer stem cells (BCSCs). These cells possess the abilities of self-renewal and tumor initiation, allowing them to be drivers of metastases and tumor growth. The microenvironment in which these cells reside is filled with residential inflammatory cells that provide the needed signaling cues for BCSC-mediated self-renewal and survival. The production of cytokines allows these cells to escape from the primary tumor and travel through the circulation to distant organs, commencing the process of metastasis. Due to their significant role in driving disease progression, BCSCs represent a new target by which to treat the tumor at the source of metastasis.

Invasion is the process by which cancer cells directly extend and penetrate into neighboring tissues in cancer. It is generally distinguished from metastasis, which is the spread of cancer cells through the circulatory system or the lymphatic system to more distant locations. Yet, lymphovascular invasion is generally the first step of metastasis.
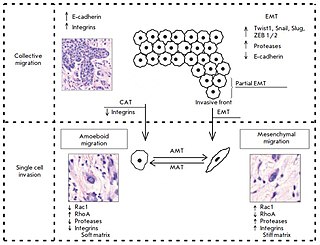
The collective–amoeboid transition (CMT) is a process by which collective multicellular groups dissociate into amoeboid single cells following the down-regulation of integrins. CMTs contrast with epithelial–mesenchymal transitions (EMT) which occur following a loss of E-cadherin. Like EMTs, CATs are involved in the invasion of tumor cells into surrounding tissues, with amoeboid movement more likely to occur in soft extracellular matrix (ECM) and mesenchymal movement in stiff ECM. Although once differentiated, cells typically do not change their migration mode, EMTs and CMTs are highly plastic with cells capable of interconverting between them depending on intracelluar regulatory signals and the surrounding ECM.














