
A Carnot heat engine is a theoretical heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates.

A heat engine is a system that converts heat to usable energy, particularly mechanical energy, which can then be used to do mechanical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.

Energy storage is the capture of energy produced at one time for use at a later time to reduce imbalances between energy demand and energy production. A device that stores energy is generally called an accumulator or battery. Energy comes in multiple forms including radiation, chemical, gravitational potential, electrical potential, electricity, elevated temperature, latent heat and kinetic. Energy storage involves converting energy from forms that are difficult to store to more conveniently or economically storable forms.

Solar energy is radiant light and heat from the Sun that is harnessed using a range of technologies such as solar power to generate electricity, solar thermal energy, and solar architecture. It is an essential source of renewable energy, and its technologies are broadly characterized as either passive solar or active solar depending on how they capture and distribute solar energy or convert it into solar power. Active solar techniques include the use of photovoltaic systems, concentrated solar power, and solar water heating to harness the energy. Passive solar techniques include orienting a building to the Sun, selecting materials with favorable thermal mass or light-dispersing properties, and designing spaces that naturally circulate air.

A heat pump is a device that uses work to transfer heat from a cool space to a warm space by transferring thermal energy using a refrigeration cycle, cooling the cool space and warming the warm space. In cold weather a heat pump can move heat from the cool outdoors to warm a house; the pump may also be designed to move heat from the house to the warmer outdoors in warm weather. As they transfer heat rather than generating heat, they are more energy-efficient than other ways of heating or cooling a home.

Carnot's theorem, also called Carnot's rule, is a principle of thermodynamics developed by Nicolas Léonard Sadi Carnot in 1824 that specifies limits on the maximum efficiency that any heat engine can obtain.

Solar thermal energy (STE) is a form of energy and a technology for harnessing solar energy to generate thermal energy for use in industry, and in the residential and commercial sectors.
A thermal reservoir, also thermal energy reservoir or thermal bath, is a thermodynamic system with a heat capacity so large that the temperature of the reservoir changes relatively little when a significant amount of heat is added or extracted. As a conceptual simplification, it effectively functions as an infinite pool of thermal energy at a given, constant temperature. Since it can act as an inertial source and sink of heat, it is often also referred to as a heat reservoir or heat bath.
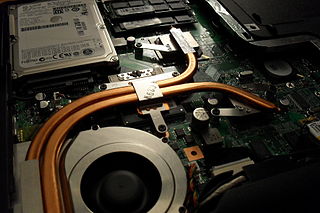
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

Solar water heating (SWH) is heating water by sunlight, using a solar thermal collector. A variety of configurations are available at varying cost to provide solutions in different climates and latitudes. SWHs are widely used for residential and some industrial applications.
The coefficient of performance or COP of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy (power) consumption and thus lower operating costs. The COP is used in thermodynamics.

Thermal energy storage (TES) is the storage of thermal energy for later reuse. Employing widely different technologies, it allows surplus thermal energy to be stored for hours, days, or months. Scale both of storage and use vary from small to large – from individual processes to district, town, or region. Usage examples are the balancing of energy demand between daytime and nighttime, storing summer heat for winter heating, or winter cold for summer cooling. Storage media include water or ice-slush tanks, masses of native earth or bedrock accessed with heat exchangers by means of boreholes, deep aquifers contained between impermeable strata; shallow, lined pits filled with gravel and water and insulated at the top, as well as eutectic solutions and phase-change materials.

A solar pond is a pool of saltwater which collects and stores solar thermal energy. The saltwater naturally forms a vertical salinity gradient also known as a "halocline", in which low-salinity water floats on top of high-salinity water. The layers of salt solutions increase in concentration with depth. Below a certain depth, the solution has a uniformly high salt concentration.
Renewable heat is an application of renewable energy referring to the generation of heat from renewable sources; for example, feeding radiators with water warmed by focused solar radiation rather than by a fossil fuel boiler. Renewable heat technologies include renewable biofuels, solar heating, geothermal heating, heat pumps and heat exchangers. Insulation is almost always an important factor in how renewable heating is implemented.
Seasonal thermal energy storage (STES), also known as inter-seasonal thermal energy storage, is the storage of heat or cold for periods of up to several months. The thermal energy can be collected whenever it is available and be used whenever needed, such as in the opposing season. For example, heat from solar collectors or waste heat from air conditioning equipment can be gathered in hot months for space heating use when needed, including during winter months. Waste heat from industrial process can similarly be stored and be used much later or the natural cold of winter air can be stored for summertime air conditioning.

A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system.

Energy recovery includes any technique or method of minimizing the input of energy to an overall system by the exchange of energy from one sub-system of the overall system with another. The energy can be in any form in either subsystem, but most energy recovery systems exchange thermal energy in either sensible or latent form.

In thermodynamics, heat is the thermal energy transferred between systems due to a temperature difference. In colloquial use, heat sometimes refers to thermal energy itself. Thermal energy is the kinetic energy of vibrating and colliding atoms in a substance.

A hot water storage tank is a water tank used for storing hot water for space heating or domestic use.
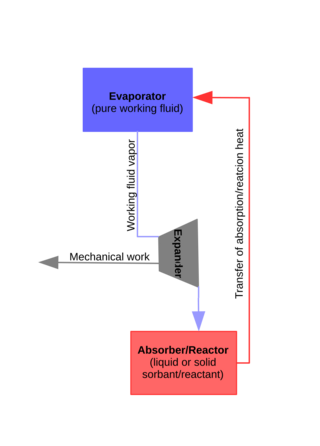
The Lamm-Honigmann process is a storage and heat to power conversion process that consists of using the effect of vapor pressure depression of a working fluid mixture compared to a pure working fluid of that mixture. This process is named after their independent inventors Emile Lamm and Moritz Honigmann. Both inventors envisioned and realized the same process principle for usage as energy storage in so-called Fireless locomotive but with different working fluid pairs: Emile Lamm used ammonia and water, Moritz Honigmann used water and caustic soda.














