Related Research Articles

Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d.

Photosynthesis is a biological process used by many cellular organisms to convert light energy into chemical energy, which is stored in organic compounds that can later be metabolized through cellular respiration to fuel the organism's activities. The term usually refers to oxygenic photosynthesis, where oxygen is produced as a byproduct, and some of the chemical energy produced is stored in carbohydrate molecules such as sugars, starch and cellulose, which are synthesized from endergonic reaction of carbon dioxide with water. Most plants, algae and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the biological energy necessary for complex life on Earth.

Carl David Anderson was an American physicist. He is best known for his discovery of the positron in 1932, an achievement for which he received the 1936 Nobel Prize in Physics, and of the muon in 1936.

C4 carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960s discovery by Marshall Davidson Hatch and Charles Roger Slack that some plants, when supplied with 14CO2, incorporate the 14C label into four-carbon molecules first.

Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal CO2 acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose called ribulose. Salts of RuBP can be isolated, but its crucial biological function happens in solution. RuBP occurs not only in plants but in all domains of life, including Archaea, Bacteria, and Eukarya.

Chloroflexus aurantiacus is a photosynthetic bacterium isolated from hot springs, belonging to the green non-sulfur bacteria. This organism is thermophilic and can grow at temperatures from 35 °C to 70 °C. Chloroflexus aurantiacus can survive in the dark if oxygen is available. When grown in the dark, Chloroflexus aurantiacus has a dark orange color. When grown in sunlight it is dark green. The individual bacteria tend to form filamentous colonies enclosed in sheaths, which are known as trichomes.

Jan Ingenhousz or Ingen-Housz FRS was a Dutch-born British physiologist, biologist and chemist.

Photosystem I is one of two photosystems in the photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane protein complex that uses light energy to catalyze the transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin. Ultimately, the electrons that are transferred by Photosystem I are used to produce the moderate-energy hydrogen carrier NADPH. The photon energy absorbed by Photosystem I also produces a proton-motive force that is used to generate ATP. PSI is composed of more than 110 cofactors, significantly more than Photosystem II.
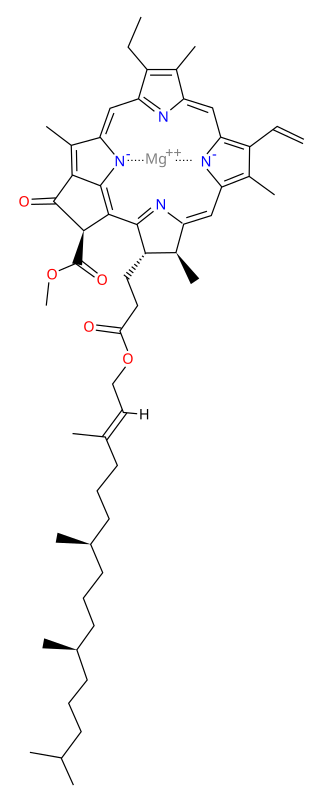
Chlorophyll a is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll a also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located.
Ferredoxins are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel. Photocatalytic water splitting converts water into hydrogen and oxygen and is a major research topic of artificial photosynthesis. Light-driven carbon dioxide reduction is another process studied that replicates natural carbon fixation.

Robert Hill FRS, known as Robin Hill, was a British plant biochemist who, in 1939, demonstrated the 'Hill reaction' of photosynthesis, proving that oxygen is evolved during the light requiring steps of photosynthesis. He also made significant contributions to the development of the Z-scheme of oxygenic photosynthesis.

Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:

The cytochrome b6f complex is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, that catalyzes the transfer of electrons from plastoquinol to plastocyanin. The reaction is analogous to the reaction catalyzed by cytochrome bc1 of the mitochondrial electron transport chain. During photosynthesis, the cytochrome b6f complex is one step along the chain that transfers electrons from Photosystem II to Photosystem I, and at the same time pumps protons into the thylakoid space, contributing to the generation of an electrochemical (energy) gradient that is later used to synthesize ATP from ADP.

2,6-Dichlorophenolindophenol is a chemical compound used as a redox dye. When oxidized, DCPIP is blue with a maximal absorption at 600 nm; when reduced, DCPIP is colorless.

Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga, or cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light.

Light-dependent reactions is jargon for certain photochemical reactions that are involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions, the first occurs at photosystem II (PSII) and the second occurs at photosystem I (PSI),

David Alan Walker was a British scientist and professor of photosynthesis in the Department of Animal and Plant Sciences (APS) at the University of Sheffield. He authored over 200 scientific publications including several books during his lifetime.
The Mehler reaction is named after Alan H. Mehler, who, in 1951, presented data to the effect that isolated chloroplasts reduce oxygen to form hydrogen peroxide. Mehler observed that the H
2O
2 formed in this way does not present an active intermediate in photosynthesis; rather, as a reactive oxygen species, it can be toxic to surrounding biological processes as an oxidizing agent. In scientific literature, the Mehler reaction often is used interchangeably with the Water-Water Cycle to refer to the formation of H
2O
2 by photosynthesis. Sensu stricto, the Water Water Cycle encompasses the Hill reaction, in which water is split to form oxygen, as well as the Mehler Reaction, in which oxygen is reduced to form H
2O
2 and, finally, the scavenging of this H
2O
2 by antioxidants to form water.
The Hill reaction is the light-driven transfer of electrons from water to Hill reagents in a direction against the chemical potential gradient as part of photosynthesis. Robin Hill discovered the reaction in 1937. He demonstrated that the process by which plants produce oxygen is separate from the process that converts carbon dioxide to sugars.
References
- David Alan Walker (2002). "'And whose bright presence' – an appreciation of Robert Hill and his reaction". Photosynthesis Research. 73 (1–3): 51–54. doi:10.1023/A:1020479620680. PMID 16245102.
- Govindjee and David Krogmann (2004). "Discoveries in Oxygenic Photosynthesis (1727–2003): A Perspective". Photosynthesis Research. 80 (1–3): 15–57. doi:10.1023/B:PRES.0000030443.63979.e6. PMID 16328809.