
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism.
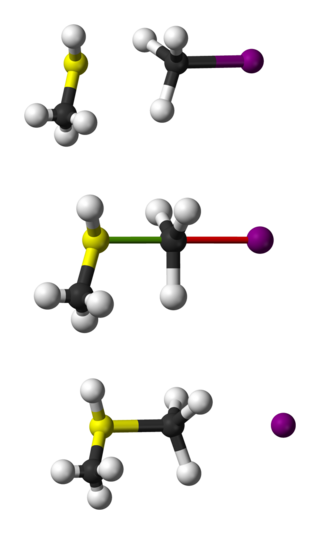
Bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted fashion.
In the Buddhist tradition, the five hindrances are identified as mental factors that hinder progress in meditation and in daily life. In the Theravada tradition, these factors are identified specifically as obstacles to the jhānas within meditation practice. Within the Mahayana tradition, the five hindrances are identified as obstacles to samatha (tranquility) meditation. Contemporary Insight Meditation teachers identify the five hindrances as obstacles to mindfulness meditation.

Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel.

In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a relatively long time period as a stable rotational isomer or rotamer. When the time scale for interconversion is long enough for isolation of individual rotamers, the isomers are termed atropisomers. The ring-flip of substituted cyclohexanes constitutes another common form of conformational isomerism.

Lithium tetramethylpiperidide is a chemical compound with the molecular formula LiC9H18N. It is used as a non-nucleophilic base, being comparable to LiHMDS in terms of steric hindrance.
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon. This chemical reaction is useful in the organic synthesis of organic compounds.
The Larock indole synthesis is a heteroannulation reaction that uses palladium as a catalyst to synthesize indoles from an ortho-iodoaniline and a disubstituted alkyne. It is also known as Larock heteroannulation. The reaction is extremely versatile and can be used to produce varying types of indoles. Larock indole synthesis was first proposed by Richard C. Larock in 1991 at Iowa State University.

N,N-Diisopropylethylamine, or Hünig's base, is an organic compound that is a tertiary amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a non-nucleophilic base. It is commonly abbreviated as DIPEA,DIEA, or i-Pr2NEt.
In chemistry, mechanically interlocked molecular architectures (MIMAs) are molecules that are connected as a consequence of their topology. This connection of molecules is analogous to keys on a keychain loop. The keys are not directly connected to the keychain loop but they cannot be separated without breaking the loop. On the molecular level, the interlocked molecules cannot be separated without the breaking of the covalent bonds that comprise the conjoined molecules; this is referred to as a mechanical bond. Examples of mechanically interlocked molecular architectures include catenanes, rotaxanes, molecular knots, and molecular Borromean rings. Work in this area was recognized with the 2016 Nobel Prize in Chemistry to Bernard L. Feringa, Jean-Pierre Sauvage, and J. Fraser Stoddart.

In chemistry, an adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can be considered as a single product resulting from the direct combination of different molecules which comprises all atoms of the reactant molecules.

Asymmetric induction describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.

An oxyanion hole is a pocket in the active site of an enzyme that stabilizes transition state negative charge on a deprotonated oxygen or alkoxide. The pocket typically consists of backbone amides or positively charged residues. Stabilising the transition state lowers the activation energy necessary for the reaction, and so promotes catalysis. For example, proteases such as chymotrypsin contain an oxyanion hole to stabilise the tetrahedral intermediate anion formed during proteolysis and protects substrate's negatively charged oxygen from water molecules. Additionally, it may allow for insertion or positioning of a substrate, which would suffer from steric hindrance if it could not occupy the hole. Enzymes that catalyse multi-step reactions can have multiple oxyanion holes that stabilise different transition states in the reaction.
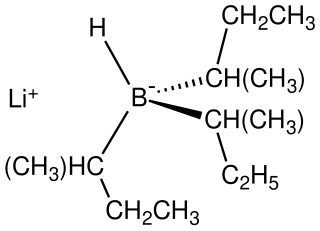
L-selectride is a organoboron compound with the chemical formula Li[(CH3CH2CH )3BH]. A colorless salt, it is usually dispensed as a solution in THF. As a particularly basic and bulky borohydride, it is used for stereoselective reduction of ketones..
A-values are numerical values used in the determination of the most stable orientation of atoms in a molecule, as well as a general representation of steric bulk. A-values are derived from energy measurements of the different cyclohexane conformations of a monosubstituted cyclohexane chemical. Substituents on a cyclohexane ring prefer to reside in the equatorial position to the axial. The difference in Gibbs free energy (ΔG) between the higher energy conformation and the lower energy conformation is the A-value for that particular substituent.
Triisopropylamine is an organic chemical compound consisting of three isopropyl groups bound to a central nitrogen atom. As a hindered tertiary amine, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis.

The White–Chen catalyst is an Iron-based coordination complex named after Professor M. Christina White and her graduate student Mark S. Chen. The catalyst is used along with hydrogen peroxide and acetic acid additive to oxidize aliphatic sp3 C-H bonds in organic synthesis. The catalyst is the first to allow for preparative and predictable aliphatic C–H oxidations over a broad range of organic substrates. Oxidations with the catalyst have proven to be remarkably predictable based on sterics, electronics, and stereoelectronics allowing for aliphatic C–H bonds to be thought of as a functional group in the streamlining of organic synthesis.
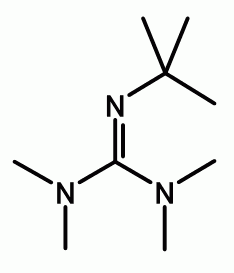
2-tert-Butyl-1,1,3,3-tetramethylguanidine is an organic base, also known as Barton's base. It is named after Nobel Prize-winning British chemist Derek Barton. Barton and his assistants prepared a series of guanidines with steric hindrance in 1982; in this case five alkyl groups: four methyl groups and one tert-butyl group. In 50% water ethanol mixture, the acidity constant (pKa) of Barton's base is 14. In acetonitrile its pKa is 24.31.
Ortho effect is an organic chemistry phenomenon where the presence of an chemical group at the at ortho position or the 1 and 2 position of a phenyl ring, relative to the carboxylic compound changes the chemical properties of the compound. This is caused by steric effects and bonding interactions along with polar effects caused by the various substituents which are in a given molecule, resulting in changes in its chemical and physical properties. The ortho effect is associated with substituted benzene compounds.
Potassium nitride is an unstable chemical compound. Several syntheses were erroneously claimed in the 19th century, and by 1894 it was assumed that it did not exist.










