Hydroperoxyeicosatetraenoic acid (HPETE) may refer to:
Hydroperoxyeicosatetraenoic acid (HPETE) may refer to:

Homocysteine or Hcy: is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine by the removal of its terminal Cε methyl group. In the body, homocysteine can be recycled into methionine or converted into cysteine with the aid of vitamin B6, B9, and B12.

Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). If its precursors or diet contains linoleic acid it is formed by biosynthesis and can be deposited in animal fats. It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes.

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Some eicosanoids, such as prostaglandins, may also have endocrine roles as hormones to influence the function of distant cells.
Lipid peroxidation, or lipid oxidation, is a complex chemical process that leads to oxidative degradation of lipids, resulting in the formation of peroxide and hydroperoxide derivatives. It occurs when free radicals, specifically reactive oxygen species (ROS), interact with lipids within cell membranes, typically polyunsaturated fatty acids (PUFAs) as they have carbon–carbon double bonds. This reaction leads to the formation of lipid radicals, collectively referred to as lipid peroxides or lipid oxidation products (LOPs), which in turn react with other oxidizing agents, leading to a chain reaction that results in oxidative stress and cell damage.

Lipoxygenases (LOX) are a family of (non-heme) iron-containing enzymes, more specifically oxidative enzymes, most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.

In evolutionary developmental biology, Paired box (Pax) genes are a family of genes coding for tissue specific transcription factors containing an N-terminal paired domain and usually a partial, or in the case of four family members, a complete homeodomain to the C-terminus. An octapeptide as well as a Pro-Ser-Thr-rich C terminus may also be present. Pax proteins are important in early animal development for the specification of specific tissues, as well as during epimorphic limb regeneration in animals capable of such.
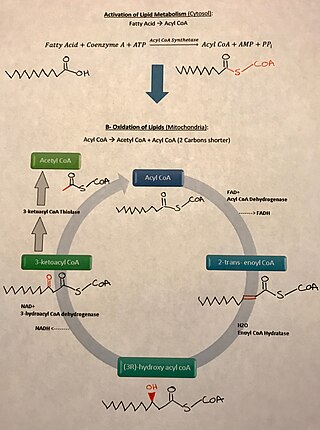
There is a wide variety of fatty acids found in nature. Two classes of fatty acids are considered essential, the omega-3 and omega-6 fatty acids. Essential fatty acids are necessary for humans but cannot be synthesized by the body and must therefore be obtained from food. Omega-3 and omega-6 are used in some cellular signaling pathways and are involved in mediating inflammation, protein synthesis, and metabolic pathways in the human body.
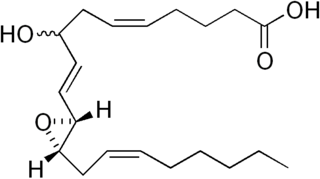
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.

Leukotriene A4 (LTA4) is a leukotriene, and is the precursor for the productions of leukotriene B4 (LTB4) and leukotriene C4 (LTC4).

Arachidonic acid 5-hydroperoxide is an intermediate in the metabolism of arachidonic acid by the ALOX5 enzyme in humans or Alox5 enzyme in other mammals. The intermediate is then further metabolized to: a) leukotriene A4 which is then metabolized to the chemotactic factor for leukocytes, leukotriene B4, or to contractors of lung airways, leukotriene C4, leukotriene D4, and leukotriene E4; b) the leukocyte chemotactic factors, 5-hydroxyicosatetraenoic acid and 5-oxo-eicosatetraenoic acid; or c) the specialized pro-resolving mediators of inflammation, lipoxin A4 and lipoxin B4.

High-mobility group protein HMG-I/HMG-Y is a protein that in humans is encoded by the HMGA1 gene.

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

G-protein coupled receptor 31 also known as 12-(S)-HETE receptor is a protein that in humans is encoded by the GPR31 gene. The human gene is located on chromosome 6q27 and encodes a G-protein coupled receptor protein composed of 319 amino acids.
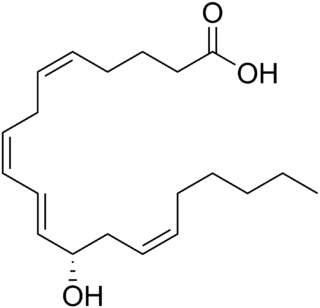
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.

WikiPathways is a community resource for contributing and maintaining content dedicated to biological pathways. Any registered WikiPathways user can contribute, and anybody can become a registered user. Contributions are monitored by a group of admins, but the bulk of peer review, editorial curation, and maintenance is the responsibility of the user community. WikiPathways is originally built using MediaWiki software, a custom graphical pathway editing tool (PathVisio) and integrated BridgeDb databases covering major gene, protein, and metabolite systems. WikiPathways was founded in 2008 by Thomas Kelder, Alex Pico, Martijn Van Iersel, Kristina Hanspers, Bruce Conklin and Chris Evelo. Current architects are Alex Pico and Martina Summer-Kutmon.
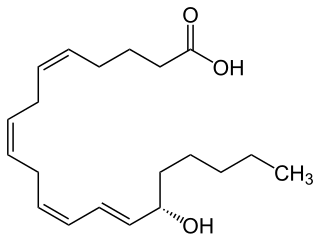
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
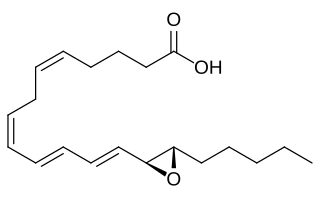
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.
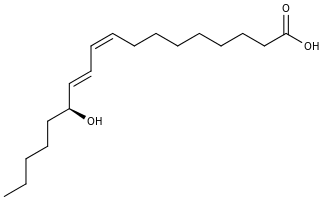
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.

9-Hydroxyoctadecadienoic acid (or 9-HODE) has been used in the literature to designate either or both of two stereoisomer metabolites of the essential fatty acid, linoleic acid: 9(S)-hydroxy-10(E),12(Z)-octadecadienoic acid (9(S)-HODE) and 9(R)-hydroxy-10(E),12(Z)-octadecadienoic acid (9(R)-HODE); these two metabolites differ in having their hydroxy residues in the S or R configurations, respectively. The accompanying figure gives the structure for 9(S)-HETE. Two other 9-hydroxy linoleic acid derivatives occur in nature, the 10E,12E isomers of 9(S)-HODE and 9(R)-HODE viz., 9(S)-hydroxy-10E,12E-octadecadienoic acid (9(S)-EE-HODE) and 9(R)-hydroxy-10E,12E-octadecadienoic acid (13(R)-EE-HODE); these two derivatives have their double bond at carbon 12 in the E or trans configuration as opposed to the Z or cis configuration. The four 9-HODE isomers, particularly under conditions of oxidative stress, may form together in cells and tissues; they have overlapping but not identical biological activities and significances. Because many studies have not distinguished between the S and R stereoisomers and, particularly in identifying tissue levels, the two EE isomers, 9-HODE is used here when the isomer studied is unclear.
Sherwood Leslie Gorbach is an Emeritus Professor at Tufts University School of Medicine. He was editor-in-chief of the journal Clinical Infectious Diseases from 2000 to 2016.