
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the fragment antigen-binding (Fab) variable region. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly. Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region, which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system.

Globular proteins or spheroproteins are spherical ("globe-like") proteins and are one of the common protein types. Globular proteins are somewhat water-soluble, unlike the fibrous or membrane proteins. There are multiple fold classes of globular proteins, since there are many different architectures that can fold into a roughly spherical shape.
A radioallergosorbent test (RAST) is a blood test using radioimmunoassay test to detect specific IgE antibodies, to determine the substances a subject is allergic to. This is different from a skin allergy test, which determines allergy by the reaction of a person's skin to different substances.

Immunoglobulin A is an antibody that plays a crucial role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined. In absolute terms, between three and five grams are secreted into the intestinal lumen each day. This represents up to 15% of total immunoglobulins produced throughout the body.
Immunoglobulin E (IgE) is a type of antibody that has only been found in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing 4 Ig-like constant domains (Cε1-Cε4). IgE's main function is immunity to parasites such as helminths like Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum.

The complement system is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promotes inflammation, and attacks the pathogen's cell membrane. It is part of the innate immune system, which is not adaptable and does not change over the course of an individual's lifetime. The complement system can, however, be recruited and brought into action by antibodies generated by the adaptive immune system.
In molecular biology, elastase is an enzyme from the class of proteases (peptidases) that break down proteins. In particular, it is a serine protease.

The adaptive immune system, also known as the acquired immune system or, more rarely, as the specific immune system, is a subsystem of the overall immune system that is composed of highly specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates. Acquired immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to subsequent encounters with that pathogen. This process of acquired immunity is the basis of vaccination. Like the innate system, the acquired system includes both humoral immunity components and cell-mediated immunity components.

The antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.

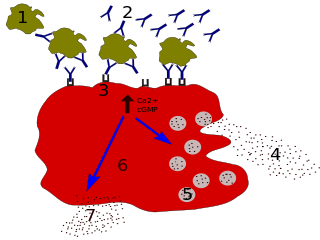
The B-cell receptor or BCR is composed of immunoglobulin molecules that form a type 1 transmembrane receptor protein usually located on the outer surface of a lymphocyte type known as B cells. Through biochemical signaling and by physically acquiring antigens from the immune synapses, the BCR controls the activation of B-cell. B cells are able to gather and grab antigens by engaging biochemical modules for receptor clustering, cell spreading, generation of pulling forces, and receptor transport, which eventually culminates in endocytosis and antigen presentation. B-cells’ mechanical activity adheres to a pattern of negative and positive feedbacks that regulate the quantity of removed antigen by manipulating the dynamic of BCR-antigen bonds directly. Particularly, grouping and spreading increase the relation of antigen with BCR, thereby proving sensitivity and amplification. On the other hand, pulling forces delinks the antigen from the BCR, thus testing the quality of antigen binding.
The fifth type of hyper-IgM syndrome has been characterized in three patients from France and Japan. The symptoms are similar to hyper IgM syndrome type 2, but the AICDA gene is intact. These three patients instead had mutations in the catalytic domain of uracil-DNA glycosylase, an enzyme that removes uracil from DNA. In both type 2 and type 5 hyper-IgM syndromes, the patients are profoundly deficient in IgG and IgA because the B cells can't carry out the recombination steps necessary to class-switch.

The antigen-binding (Fab) fragment is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope, comprising a set of complementarity determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen.

6-Phosphogluconate dehydrogenase (6PGD) is an enzyme in the pentose phosphate pathway. It forms ribulose 5-phosphate from 6-phosphogluconate.

Immunoglobulin class switching, also known as isotype switching, isotypic commutation or class-switch recombination (CSR), is a biological mechanism that changes a B cell's production of immunoglobulin from one type to another, such as from the isotype IgM to the isotype IgG. During this process, the constant-region portion of the antibody heavy chain is changed, but the variable region of the heavy chain stays the same. Since the variable region does not change, class switching does not affect antigen specificity. Instead, the antibody retains affinity for the same antigens, but can interact with different effector molecules.

In immunology, the immunoglobulin (Ig) isotype (class) is encoded by the constant region segments of the immunoglobulin gene which form the Fc portion of an antibody. The expression of a specific isotype determines the function of an antibody via the specific binding to Fc receptor molecules on different immune effector cells. Isotype expression reflects the maturation stage of a B cell. Naive B cells express IgM and IgD isotypes with unmutated variable genes, which are produced from the same initial transcript following alternative splicing. Expression of other antibody isotypes occurs via a process of class-switch recombination (CSR) after antigen exposure. Class-switching is mediated by the AID enzyme and only occurs after the B cell binds an antigen through its B cell receptor, and is further activated through interaction with a T helper cell.

Protein-glutamine gamma-glutamyltransferase K is an enzyme that in humans is encoded by the TGM1 gene.
In enzymology, a thiamine diphosphokinase is an enzyme that catalyzes the chemical reaction
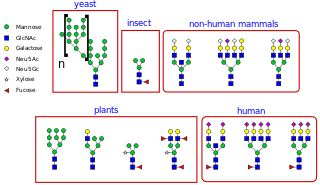
N-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. This type of linkage is important for both the structure and function of some eukaryotic proteins. The N-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of N-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of N-linked glycan.















