| Look up Iodine or iodine in Wiktionary, the free dictionary. |
Iodine is a chemical element with symbol I and atomic number 53.

Iodine is a chemical element with symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a lustrous, purple-black non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius. The element was discovered by the French chemist Bernard Courtois in 1811. It was named two years later by Joseph Louis Gay-Lussac from this property, after the Greek ἰώδης "violet-coloured".
Iodine may also refer to:
- Isotopes of iodine:
Iodine-129 (129I) is a long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant.
Iodine-131 (131I) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because I-131 is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. I-131 is also a major fission product of uranium-233, produced from thorium.
- Iodine clock reaction
- Iodine (medical use)
- Povidone-iodine, a common antiseptic
- Tincture of iodine
- Lugol's iodine

Povidone-iodine (PVP-I), also known as iodopovidone, is an antiseptic used for skin disinfection before and after surgery. It may be used both to disinfect the skin of the patient and the hands of the healthcare providers. It may also be used for minor wounds. It may be applied to the skin as a liquid or a powder.
Tincture of iodine, iodine tincture, or weak iodine solution is an antiseptic. It is usually 2–7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water. Tincture solutions are characterized by the presence of alcohol. It was used from 1908 in pre-operative skin preparation by surgeon Antonio Grossich.

Lugol's iodine, also known as aqueous iodine and strong iodine solution, is a solution of potassium iodide with iodine in water. It is a medication and disinfectant used for a number of purposes. Taken by mouth it is used to treat thyrotoxicosis until surgery can be carried out, protect the thyroid gland from radioactive iodine, and to treat iodine deficiency. When applied to the cervix it is used to help in screening for cervical cancer. As a disinfectant it may be applied to small wounds such as a needle stick injury. A small amount may also be used for emergency disinfection of drinking water.
- Iodine deficiency
- Iodine Recordings
- Iodine test
- Iodine value
- Little Iodine, a comics character
- "Iodine", a song by Leonard Cohen from Death of a Ladies' Man
- Iodine (film), a 2009 Canadian science-fiction film
- "Iodine", a song by Icon For Hire from Scripted
There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
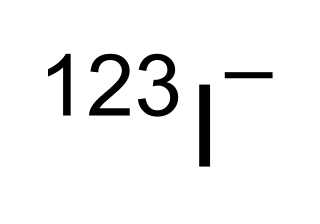
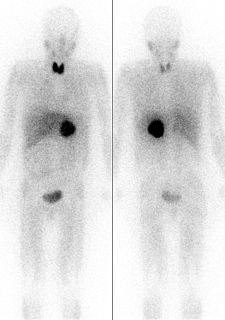
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.22 hours; the decay by electron capture to tellurium-123 emits gamma radiation with a predominant energy of 159 keV. In medical applications, the radiation is detected by a gamma camera. The isotope is typically applied as iodide-123, the anionic form.
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.