
Manganese is a chemical element with the symbol Mn and atomic number 25. It is found as a free element in nature; it is often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels.

Manganese(IV) oxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc-carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 in the α polymorph can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium ion batteries.
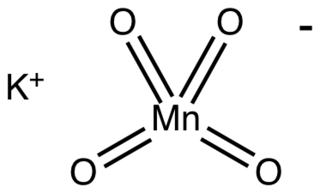
Potassium permanganate is an inorganic compound with the chemical formula KMnO4 and composed of K+ and MnO−
4. It is a purplish-black crystalline solid, that dissolves in water to give intensely pink or purple solutions.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. They are manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All known elements of group 7 are transition metals.
Manganese oxide is any of a variety of manganese oxides and hydroxides. These include
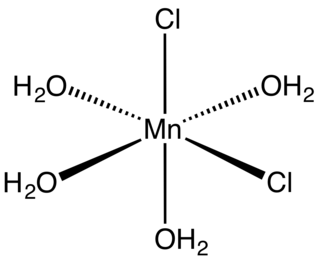
Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

A permanganate is the general name for a chemical compound containing the manganate(VII) ion, (MnO−
4). Because manganese is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion has tetrahedral geometry. Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media. The exact chemical reaction is dependent upon the organic contaminants present and the oxidant utilized. For example, trichloroethane (C2H3Cl3) is oxidized by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

A zinc–carbon battery is a dry cell primary battery that delivers about 1.5 volts of direct current from the electrochemical reaction between zinc and manganese dioxide. A carbon rod collects the current from the manganese dioxide electrode, giving the name to the cell. A dry cell is usually made from zinc, which serves as the anode with a negative electrical polarity, while the inert carbon rod is the positive electrical pole cathode. General-purpose batteries may use an aqueous paste of ammonium chloride as electrolyte, possibly mixed with some zinc chloride solution. Heavy-duty types use a paste primarily composed of zinc chloride.
Naturally occurring manganese (25Mn) is composed of one stable isotope, 55Mn. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3.7 million years, 54Mn with a half-life of 312.3 days, and 52Mn with a half-life of 5.591 days. All of the remaining radioactive isotopes have half-lives that are less than 3 hours and the majority of these have half-lives that are less than a minute, but only 45Mn has an unknown half-life. The least stable is 44Mn with a half-life shorter than 105 nanoseconds. This element also has 3 meta states.
Potassium manganate is the inorganic compound with the formula K2MnO4. This green-colored salt is an intermediate in the industrial synthesis of potassium permanganate (KMnO4), a common chemical. Occasionally, potassium manganate and potassium permanganate are confused, but they are different compounds with distinctly different properties.

Manganese(VII) oxide (manganese heptoxide) is an inorganic compound with the formula Mn2O7. This volatile liquid is highly reactive and more often discussed than intentionally prepared. It is a dangerous oxidizer and was first described in 1860. It is the acid anhydride of permanganic acid.
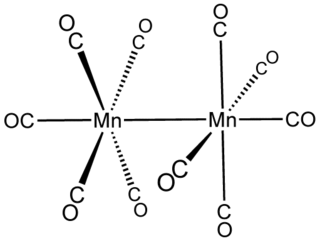
Dimanganese decacarbonyl is the chemical compound with the formula Mn2(CO)10. This metal carbonyl is an important reagent in the organometallic chemistry of manganese.

Manganese(II) sulfate usually refers to the inorganic compound with the formula MnSO4·H2O. This pale pink deliquescent solid is a commercially significant manganese(II) salt. Approximately 260,000 tonnes of manganese(II) sulfate were produced worldwide in 2005. It is the precursor to manganese metal and many other chemical compounds. Manganese-deficient soil is remediated with this salt.
Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO.Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.

Manganese(II) oxide is an inorganic compound with chemical formula MnO. It forms green crystals. The compound is produced on a large scale as a component of fertilizers and food additives.

Manganese(III) oxide is a chemical compound with the formula Mn2O3.

Manganese(II) sulfide is a chemical compound of manganese and sulfur. It occurs in nature as the mineral alabandite (isometric), rambergite (hexagonal), and recently found browneite.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a recent review Cahiez et al. argue that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.

Manganese(II) nitrate are the inorganic compounds with formula Mn(NO3)2(H2O)n. Each formula unit is composed of one Mn2+ cation and two NO3− anions and varying amounts of water. Most common is the tetrahydrate Mn(NO3)2·4H2O, but mono- and hexahydrates are also known as well as the anhydrous compound. Some of these compounds are useful precursors to the oxides of manganese.















