
Methylphenethylamine may refer to:
- α-Methylphenethylamine (amphetamine)
- β-Methylphenethylamine
- N-Methylphenethylamine (an endogenous trace amine in humans)
- 2-Methylphenethylamine
- 3-Methylphenethylamine
- 4-Methylphenethylamine

Methylphenethylamine may refer to:
| | This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as monochloramine (NClH2).

Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
A biogenic amine is a biogenic substance with one or more amine groups. They are basic nitrogenous compounds formed mainly by decarboxylation of amino acids or by amination and transamination of aldehydes and ketones. Biogenic amines are organic bases with low molecular weight and are synthesized by microbial, vegetable and animal metabolisms. In food and beverages they are formed by the enzymes of raw material or are generated by microbial decarboxylation of amino acids.

Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems.

β-Methylphenethylamine is an organic compound of the phenethylamine class, and a positional isomer of the drug amphetamine, with which it shares some properties. In particular, both amphetamine and β-methylphenethylamine are human TAAR1 agonists. In appearance, it is a colorless or yellowish liquid.
Phenylethanolamine N-methyltransferase (PNMT) is an enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline). It is also expressed in small groups of neurons in the human brain and in select populations of cardiomyocytes.

Senegalia berlandieri is a shrub native to the Southwestern United States and northeast Mexico that belongs to the subfamily Mimosoideae (wattles) of Fabaceae (legumes). It grows 1 to 5 metres tall, with blossoms that are spherical and white, occurring from February through April. The berlandieri epithet comes from the name of Jean-Louis Berlandier, a French naturalist who studied wildlife native to Texas and Mexico. S. berlandieri contains a wide variety of alkaloids and has been known to cause toxic reactions in domestic animals such as goats.

Vachellia rigidula, commonly known as blackbrush acacia or chaparro prieto, and also known as Acacia rigidula, is a species of shrub or small tree in the legume family, Fabaceae. Its native range stretches from Texas in the United States south to central Mexico. This perennial is not listed as being threatened. It reaches a height of 5–15 feet (1.5–4.6 m). Blackbrush Acacia grows on limestone hillsides and canyons.

N-Methylphenethylamine (NMPEA) is a naturally occurring trace amine neuromodulator in humans that is derived from the trace amine, phenethylamine (PEA). It has been detected in human urine and is produced by phenylethanolamine N-methyltransferase with phenethylamine as a substrate. PEA and NMPEA are both alkaloids that are found in a number of different plant species as well. Some Acacia species, such as A. rigidula, contain remarkably high levels of NMPEA. NMPEA is also present at low concentrations in a wide range of foodstuffs.

Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the TAAR1 gene. TAAR1 is an intracellular amine-activated Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells, astrocytes, and in the intracellular milieu within the presynaptic plasma membrane of monoamine neurons in the central nervous system (CNS). TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski et al. and Bunzow et al. TAAR1 is one of six functional human trace amine-associated receptors, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmune system function through different mechanisms.
The molecular formula C9H13N may refer to:

Homarylamine is a substituted phenethylamine. It is the N-methylated analog of MDPEA. Homoarylamine was patented as an antitussive in 1956, but has never been used medically as such.

N-Methyltyramine (NMT), also known as 4-hydroxy-N-methylphenethylamine, is a human trace amine and natural phenethylamine alkaloid found in a variety of plants. As the name implies, it is the N-methyl analog of tyramine, which is a well-known biogenic trace amine with which NMT shares many pharmacological properties. Biosynthetically, NMT is produced by the N-methylation of tyramine via the action of the enzyme phenylethanolamine N-methyltransferase in humans and tyramine N-methyltransferase in plants.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.

N,N-Dimethyldopamine (DMDA) is an organic compound belonging to the phenethylamine family. It is related structurally to the alkaloid epinine (N-methyldopamine) and to the major neurotransmitter dopamine. Because of its structural relationship to dopamine, DMDA has been the subject of a number of pharmacological investigations. DMDA has been detected in Acacia rigidula.
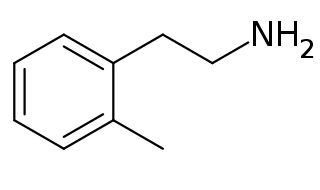
2-Methylphenethylamine (2MPEA) is an organic compound with the chemical formula of C9H13N. 2MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine (a trace amine).

3-Methylphenethylamine (3MPEA) is an organic compound with the chemical formula of C9H13N. 3MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine (a trace amine).

4-Methylphenethylamine (4MPEA), also known as para-methylphenethylamine, is an organic compound with the chemical formula of C9H13N. 4MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine (a trace amine). 4MPEA also appears to inhibit the human cytochrome P450 enzymes CYP1A2 and CYP2A6, based upon the published literature.

2-Phenyl-3-aminobutane is a stimulant of the phenethylamine class that is closely related to its α-methyl analog Pentorex. It was first synthesized by the German scientists Felix Haffner and Fritz Sommer in 1939 as a stimulant with milder effects, shorter duration, lower toxicity and fewer side effects compared to previously known drugs such as amphetamine.