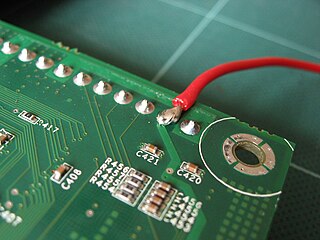
Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. The word solder comes from the Middle English word soudur, via Old French solduree and soulder, from the Latin solidare, meaning "to make solid". In fact, solder must first be melted in order to adhere to and connect the pieces together after cooling, which requires that an alloy suitable for use as solder have a lower melting point than the pieces being joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

Sintering is the process of compacting and forming a solid mass of material by heat or pressure without melting it to the point of liquefaction.

Corrosion is a natural process, which converts a refined metal to a more chemically-stable form, such as its oxide, hydroxide, or sulfide. It is the gradual destruction of materials by chemical and/or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and stopping corrosion.

Heat treating is a group of industrial and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. It is noteworthy that while the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.
In materials science, superplasticity is a state in which solid crystalline material is deformed well beyond its usual breaking point, usually over about 200% during tensile deformation. Such a state is usually achieved at high homologous temperature. Examples of superplastic materials are some fine-grained metals and ceramics. Other non-crystalline materials (amorphous) such as silica glass and polymers also deform similarly, but are not called superplastic, because they are not crystalline; rather, their deformation is often described as Newtonian fluid. Superplastically deformed material gets thinner in a very uniform manner, rather than forming a "neck" that leads to fracture. Also, the formation of microvoids, which is another cause of early fracture, is inhibited.

Brazing is a metal-joining process in which two or more metal items are joined together by melting and flowing a filler metal into the joint, the filler metal having a lower melting point than the adjoining metal.

A material is brittle if, when subjected to stress, it breaks without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. Breaking is often accompanied by a snapping sound. Brittle materials include most ceramics and glasses and some polymers, such as PMMA and polystyrene. Many steels become brittle at low temperatures, depending on their composition and processing.

In materials science, quenching is the rapid cooling of a workpiece in water, oil or air to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring. It does this by reducing the window of time during which these undesired reactions are both thermodynamically favorable, and kinetically accessible; for instance, quenching can reduce the crystal grain size of both metallic and plastic materials, increasing their hardness.
In materials science, fracture toughness is a property which describes the ability of a material to resist fracture, and is one of the most important properties of any material for many design applications. The linear-elastic fracture toughness of a material is determined from the stress intensity factor at which a thin crack in the material begins to grow. It is denoted KIc and has the units of
or
. Plastic-elastic fracture toughness is denoted by JIc, with the unit of J/cm2 or lbf-in/in2, and is a measurement of the energy required to grow a thin crack.
Annealing, in metallurgy and materials science, is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for a suitable amount of time, and then cooling.
Hardness is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. Some materials are harder than others. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, there are different measurements of hardness: scratch hardness, indentation hardness, and rebound hardness.

Electrical steel is an iron alloy tailored to produce specific magnetic properties: small hysteresis area resulting in low power loss per cycle, low core loss, and high permeability.

Ceramic engineering is the science and technology of creating objects from inorganic, non-metallic materials. This is done either by the action of heat, or at lower temperatures using precipitation reactions from high-purity chemical solutions. The term includes the purification of raw materials, the study and production of the chemical compounds concerned, their formation into components and the study of their structure, composition and properties.

Space weathering is the type of weathering that occurs to any object exposed to the harsh environment of outer space. Bodies without atmospheres take on many weathering processes:
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.

Ultrasonic machining, or strictly speaking the "Ultrasonic vibration machining", is a subtraction manufacturing process that removes material from the surface of a part through high frequency, low amplitude vibrations of a tool against the material surface in the presence of fine abrasive particles. The tool travels vertically or orthogonal to the surface of the part at amplitudes of 0.05 to 0.125 mm. The fine abrasive grains are mixed with water to form a slurry that is distributed across the part and the tip of the tool. Typical grain sizes of the abrasive material range from 100 to 1000, where smaller grains produce smoother surface finishes.

Solid is one of the four fundamental states of matter. In solids particles are closely packed. It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a gas does. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because in gases molecules are loosely packed.

Cemented carbide is a hard material used extensively as cutting tool material, as well as other industrial applications. It consists of fine particles of carbide cemented into a composite by a binder metal. Cemented carbides commonly use tungsten carbide (WC), titanium carbide (TiC), or tantalum carbide (TaC) as the aggregate. Mentions of "carbide" or "tungsten carbide" in industrial contexts usually refer to these cemented composites.
















