This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.
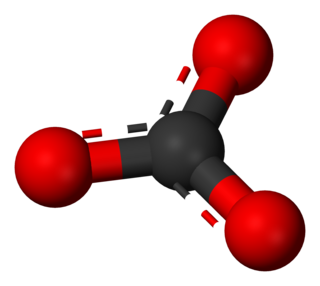
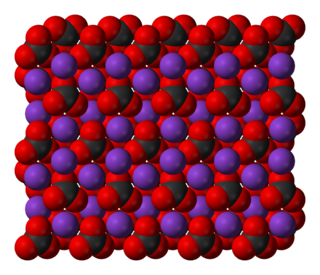
In chemistry, a carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO2−
3. The name may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.

In chemistry, a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Salts are composed of related numbers of cations and anions so that the product is electrically neutral. These component ions can be inorganic, such as chloride (Cl−), or organic, such as acetate ; and can be monatomic, such as fluoride (F−), or polyatomic, such as sulfate.
Saltpeter, Salpeter or saltpetre may refer to:

Potassium nitrate is a chemical compound with the chemical formula KNO3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate.

Natron is a naturally occurring mixture of sodium carbonate decahydrate (Na2CO3·10H2O, a kind of soda ash) and around 17% sodium bicarbonate (also called baking soda, NaHCO3) along with small quantities of sodium chloride and sodium sulfate. Natron is white to colourless when pure, varying to gray or yellow with impurities. Natron deposits are sometimes found in saline lake beds which arose in arid environments. Throughout history natron has had many practical applications that continue today in the wide range of modern uses of its constituent mineral components.
A primary standard in metrology is a standard that is sufficiently accurate such that it is not calibrated by or subordinate to other standards. Primary standards are defined via other quantities like length, mass and time. Primary standards are used to calibrate other standards referred to as working standards. See Hierarchy of Standards.

Niter, or nitre (chiefly British), is the mineral form of potassium nitrate, KNO3, also known as saltpeter or saltpetre. Historically, the term niter was not well differentiated from natron, both of which have been very vaguely defined but generally refer to compounds of sodium or potassium joined with carbonate or nitrate ions.

Caliche is a sedimentary rock, a hardened natural cement of calcium carbonate that binds other materials—such as gravel, sand, clay, and silt. It occurs worldwide, in aridisol and mollisol soil orders—generally in arid or semiarid regions, including in central and western Australia, in the Kalahari Desert, in the High Plains of the western USA, in the Sonoran Desert and Mojave Desert, and in Eastern Saudi Arabia Al-Hasa. Caliche is also known as calcrete or kankar. It belongs to the duricrusts. The term caliche is Spanish and is originally from the Latin calx, meaning lime.

Natrocarbonatite is a rare carbonatite lava which erupts from the Ol Doinyo Lengai volcano in Tanzania within the East African Rift of eastern Africa.

Nitratine or nitratite, also known as cubic niter (UK: nitre), soda niter or Chile saltpeter (UK: Chile saltpetre), is a mineral, the naturally occurring form of sodium nitrate, NaNO3. Chemically it is the sodium analogue of saltpeter. Nitratine crystallizes in the trigonal system, but rarely occurs as well formed crystals. It is isostructural with calcite. It is quite soft and light with a Mohs hardness of 1.5 to 2 and a specific gravity of 2.24 to 2.29. Its refractive indices are nω=1.587 and nε=1.336.

Potassium chromate is the inorganic compound with the formula (K2CrO4). This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially. It is a class two carcinogen as it contains hexavalent chromium.
Salpeter, Salpetre or Saltpeter most commonly refers to Nitre, KNO3.

Mineral ascorbates are a group of salts of ascorbic acid. They are composed of a mineral cation bonded to ascorbate.

Manganese(II) sulfate usually refers to the inorganic compound with the formula MnSO4·H2O. This pale pink deliquescent solid is a commercially significant manganese(II) salt. Approximately 260 thousand tonnes of manganese(II) sulfate were produced worldwide in 2005. It is the precursor to manganese metal and many other chemical compounds. Mn-deficient soil is remediated with this salt.

Chemische Fabrik Kalk (CFK) was a German chemicals company based in Kalk, a city district of Cologne. The company was founded in 1858 as Chemische Fabrik Vorster & Grüneberg, Cöln by Julius Vorster and Hermann Julius Grüneberg and was renamed to Chemische Fabrik Kalk GmbH in 1892. At times the company was the second-largest German producer of soda ash and was, with almost 2400 employees, one of the largest employers in Cologne. For decades the chimneys and the water tower of the factory dominated the skyline of Cologne-Kalk.

Uranyl carbonate, UO2(CO3), is a carbonate of uranium that forms the backbone of several uranyl mineral species such as andersonite, mckelveyite-(Y) and wyartite and most importantly rutherfordine. It is also found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water.