
β-galactosidase, also called lactase, beta-gal or β-gal, is a glycoside hydrolase enzyme that catalyzes the hydrolysis of β-galactosides into monosaccharides through the breaking of a glycosidic bond. β-galactosides include carbohydrates containing galactose where the glycosidic bond lies above the galactose molecule. Substrates of different β-galactosidases include ganglioside GM1, lactosylceramides, lactose, and various glycoproteins.

Mannose, packaged as the nutritional supplement "d-mannose", is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism.

The lac operon is an operon required for the transport and metabolism of lactose in Escherichia coli and many other enteric bacteria. Although glucose is the preferred carbon source for most bacteria, the lac operon allows for the effective digestion of lactose when glucose is not available through the activity of beta-galactosidase. Gene regulation of the lac operon was the first genetic regulatory mechanism to be understood clearly, so it has become a foremost example of prokaryotic gene regulation. It is often discussed in introductory molecular and cellular biology classes for this reason. This lactose metabolism system was used by François Jacob and Jacques Monod to determine how a biological cell knows which enzyme to synthesize. Their work on the lac operon won them the Nobel Prize in Physiology in 1965.
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known as multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm. The PTS system uses active transport. After the translocation across the membrane, the metabolites transported are modified. The system was discovered by Saul Roseman in 1964. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) transports and phosphorylates its sugar substrates in a single energy-coupled step. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB)) as well as the integral membrane sugar permease (IIC).The PTS Enzyme II complexes are derived from independently evolving 4 PTS Enzyme II complex superfamilies, that include the (1) Glucose (Glc),(2) Mannose (Man), (3) Ascorbate-Galactitol (Asc-Gat) and (4) Dihydroxyacetone (Dha) superfamilies.

Isopropyl β-D-1-thiogalactopyranoside (IPTG) is a molecular biology reagent. This compound is a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon, and it is therefore used to induce protein expression where the gene is under the control of the lac operator.
The permeases are membrane transport proteins, a class of multipass transmembrane proteins that allow the diffusion of a specific molecule in or out of the cell in the direction of a concentration gradient, a form of facilitated diffusion.

Galactoside permease is a protein coded by the lacY gene of the lac operon, and is found bound to the membrane of a cell for the purpose of binding galactoside molecules that have been solubilized. The protein is part of a system whose main function is to catalyze the accumulation and transport of lactose and other beta-galactosides across the permeable barrier of a membrane.
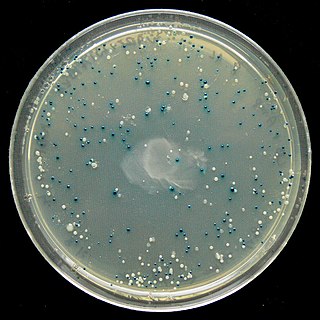
The blue–white screen is a screening technique that allows for the rapid and convenient detection of recombinant bacteria in vector-based molecular cloning experiments. DNA of interest is ligated into a vector. The vector is then inserted into a competent host cell viable for transformation, which are then grown in the presence of X-gal. Cells transformed with vectors containing recombinant DNA will produce white colonies; cells transformed with non-recombinant plasmids grow into blue colonies. This method of screening is usually performed using a suitable bacterial strain, but other organisms such as yeast may also be used.
Virulence-related outer membrane proteins are expressed in Gram-negative bacteria and are essential to bacterial survival within macrophages and for eukaryotic cell invasion.

Lactose permease is a membrane protein which is a member of the major facilitator superfamily. Lactose permease can be classified as a symporter, which uses the proton gradient towards the cell to transport β-galactosides such as lactose in the same direction into the cell.

The bacterial phosphoenolpyruvate: sugar phosphotransferase system (PTS) is a multi-protein system involved in the regulation of a variety of metabolic and transcriptional processes. The PTS catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane. The general mechanism of the PTS is the following: a phosphoryl group from phosphoenolpyruvate (PEP) is transferred to enzyme-I (EI) of PTS which in turn transfers it to a phosphoryl carrier protein (HPr). Phospho-HPr then transfers the phosphoryl group to a sugar-specific permease which consists of at least three structurally distinct domains which can either be fused together in a single polypeptide chain or exist as two or three interactive chains, formerly called enzymes II (EII) and III (EIII). The IIC domain catalyzes the transfer of a phosphoryl group from IIB to the sugar substrate.
Howard Ronald Kaback is an American Biochemist, known for Kabackosomes, the cell-free membrane transport vesicles. He is the brother of Michael M. Kaback, pediatrician and human geneticist, who developed a screening program to detect and prevent Tay–Sachs disease, a rare and fatal genetic disorder most common in Ashkenazi Jews.
The phosphotransferases system (PTS-GFL) superfamily is a superfamily of phosphotransferase enzymes that facilitate the transport of glucose, glucitol (G), fructose (F) and lactose (L). Classification has been established through phylogenic analysis and bioinformatics.
The PTSGlucose-Glucoside (Glc) family includes porters specific for glucose, glucosamine, N-acetylglucosamine and a large variety of α- and β-glucosides, and is part of the PTS-GFL superfamily.
The PTS Fructose-Mannitol (Fru) Family is a large and complex family that is part of the PTS-GFL superfamily. It includes several sequenced fructose, mannose and mannitol-specific porters, as well as several putative PTS porters of unknown specificities. The fructose porters of this family phosphorylate fructose on the 1-position. Those of TC family 4.A.6 phosphorylate fructose on the 6-position.
The PTS Glucitol (Gut) Family consists only of glucitol-specific porters, but these occur both in Gram-negative and Gram-positive bacteria. It is part of the PTS-GFL superfamily.
Permease of phosphotransferase system is a superfamily of phosphotransferase enzymes that facilitate the transport of L-ascorbate (A) and galactitol (G). Classification has been established through phylogenic analysis and bioinformatics.
The PTS Galactitol (Gat) Family is part of the PTS-AG superfamily. The biochemistry of this family is poorly defined. The only well-characterized member of this family is the galactitol permease of Escherichia coli. However, a homologous IIC protein from Listeria monocytogenes has been shown to be required for D-arabitol fermentation. It presumably functions together with IIAGat and IIBGat homologues. IICGat is distantly related to IICSgc of E. coli; IIAGat is distantly related to IIASga and IIASgcof E. coli as well as IIAMtl and IIAFru. IIBGat is distantly related to IIBSga and IIBSgc of E. coli. Domains in the LicR/CelR family of transcriptional activators show C-terminal domains exhibiting weak sequence similarity to IIBGat and IIAGat.
The PTS L-Ascorbate (L-Asc) Family includes porters specific for L-ascorbate, and is part of the PTS-AG superfamily. A single PTS permease of the L-Asc family of PTS permeases has been functionally characterized. This is the SgaTBA system, renamed UlaABC by Yew and Gerlt.
The PTS Mannose-Fructose-Sorbose (Man) Family is a group of multicomponent PTS systems that are involved in sugar uptake in bacteria. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB) as well as the integral membrane sugar permease complex (IICD). It is not part of the PTS-AG or PTS-GFL superfamilies.














