In condensed matter physics and materials science, an amorphous solid is a solid that lacks the long-range order that is characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymously with amorphous solid; however, these terms refer specifically to amorphous materials that undergo a glass transition. Examples of amorphous solids include glasses, metallic glasses, and certain types of plastics and polymers.

X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information.
Solid-state chemistry, also sometimes referred as materials chemistry, is the study of the synthesis, structure, and properties of solid phase materials. It therefore has a strong overlap with solid-state physics, mineralogy, crystallography, ceramics, metallurgy, thermodynamics, materials science and electronics with a focus on the synthesis of novel materials and their characterization. A diverse range of synthetic techniques, such as the ceramic method and chemical vapour depostion, make solid-state materials. Solids can be classified as crystalline or amorphous on basis of the nature of order present in the arrangement of their constituent particles. Their elemental compositions, microstructures, and physical properties can be characterized through a variety of analytical methods.

Zirconium dioxide is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
In metallurgy, a shape-memory alloy (SMA) is an alloy that can be deformed when cold but returns to its pre-deformed ("remembered") shape when heated. It is also known in other names such as memory metal, memory alloy, smart metal, smart alloy, and muscle wire. The "memorized geometry" can be modified by fixating the desired geometry and subjecting it to a thermal treatment, for example a wire can be taught to memorize the shape of a coil spring.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

Maraging steels are steels that are known for possessing superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of very-low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt% nickel. Secondary alloying elements, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates. Original development was carried out on 20 and 25 wt% Ni steels to which small additions of aluminium, titanium, and niobium were made; a rise in the price of cobalt in the late 1970s led to the development of cobalt-free maraging steels.
Friction welding (FWR) is a solid-state welding and bonding process that generates heat through mechanical friction between workpieces in relative motion to one another. This process is used with the addition of a lateral force called "upset" to plastically displace and fuse the materials. No melting occurs, friction welding is not a fusion welding process, but a solid-state welding technique more like forge welding. Friction welding is used with metals and thermoplastics in a wide variety of aviation and automotive applications.

Inconel is a nickel-chromium-based superalloy often utilized in extreme environments where components are subjected to high temperature, pressure or mechanical loads. Inconel alloys are oxidation- and corrosion-resistant; when heated, Inconel forms a thick, stable, passivating oxide layer protecting the surface from further attack. Inconel retains strength over a wide temperature range, attractive for high-temperature applications where aluminum and steel would succumb to creep as a result of thermally-induced crystal vacancies. Inconel's high-temperature strength is developed by solid solution strengthening or precipitation hardening, depending on the alloy.
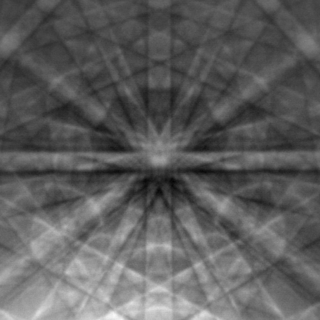
Electron backscatter diffraction (EBSD) is a scanning electron microscopy (SEM) technique used to study the crystallographic structure of materials. EBSD is carried out in a scanning electron microscope equipped with an EBSD detector comprising at least a phosphorescent screen, a compact lens and a low-light camera. In this configuration, the SEM incident beam hits the tilted sample. As backscattered electrons leave the sample, they interact with the crystal's periodic atomic lattice planes and diffract according to Bragg's law at various scattering angles before reaching the phosphor screen forming Kikuchi patterns (EBSPs). EBSD spatial resolution depends on many factors, including the nature of the material under study and the sample preparation. Thus, EBSPs can be indexed to provide information about the material's grain structure, grain orientation, and phase at the micro-scale. EBSD is applied for impurities and defect studies, plastic deformation, and statistical analysis for average misorientation, grain size, and crystallographic texture. EBSD can also be combined with energy-dispersive X-ray spectroscopy (EDS), cathodoluminescence (CL), and wavelength-dispersive X-ray spectroscopy (WDS) for advanced phase identification and materials discovery.
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope (TEM). It can involve the use of high-resolution transmission electron microscopy images, electron diffraction patterns including convergent-beam electron diffraction or combinations of these. It has been successful in determining some bulk structures, and also surface structures. Two related methods are low-energy electron diffraction which has solved the structure of many surfaces, and reflection high-energy electron diffraction which is used to monitor surfaces often during growth.
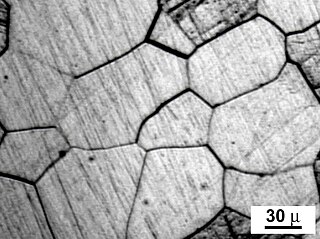
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional defects in the crystal structure, and tend to decrease the electrical and thermal conductivity of the material. Most grain boundaries are preferred sites for the onset of corrosion and for the precipitation of new phases from the solid. They are also important to many of the mechanisms of creep. On the other hand, grain boundaries disrupt the motion of dislocations through a material, so reducing crystallite size is a common way to improve mechanical strength, as described by the Hall–Petch relationship.

Crystal twinning occurs when two or more adjacent crystals of the same mineral are oriented so that they share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals that are tightly bonded to each other. The surface along which the lattice points are shared in twinned crystals is called a composition surface or twin plane.

Natural fibers or natural fibres are fibers that are produced by geological processes, or from the bodies of plants or animals. They can be used as a component of composite materials, where the orientation of fibers impacts the properties. Natural fibers can also be matted into sheets to make paper or felt.
Pseudoelasticity, sometimes called superelasticity, is an elastic (reversible) response to an applied stress, caused by a phase transformation between the austenitic and martensitic phases of a crystal. It is exhibited in shape-memory alloys.
Nanogeoscience is the study of nanoscale phenomena related to geological systems. Predominantly, this is investigated by studying environmental nanoparticles between 1–100 nanometers in size. Other applicable fields of study include studying materials with at least one dimension restricted to the nanoscale and the transfer of energy, electrons, protons, and matter across environmental interfaces.

Crystallographic image processing (CIP) is traditionally understood as being a set of key steps in the determination of the atomic structure of crystalline matter from high-resolution electron microscopy (HREM) images obtained in a transmission electron microscope (TEM) that is run in the parallel illumination mode. The term was created in the research group of Sven Hovmöller at Stockholm University during the early 1980s and became rapidly a label for the "3D crystal structure from 2D transmission/projection images" approach. Since the late 1990s, analogous and complementary image processing techniques that are directed towards the achieving of goals with are either complementary or entirely beyond the scope of the original inception of CIP have been developed independently by members of the computational symmetry/geometry, scanning transmission electron microscopy, scanning probe microscopy communities, and applied crystallography communities.
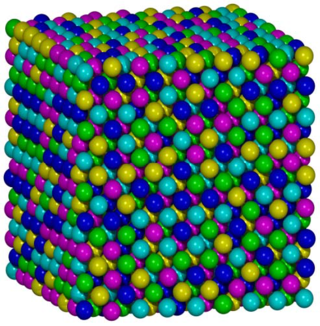
High-entropy alloys (HEAs) are alloys that are formed by mixing equal or relatively large proportions of (usually) five or more elements. Prior to the synthesis of these substances, typical metal alloys comprised one or two major components with smaller amounts of other elements. For example, additional elements can be added to iron to improve its properties, thereby creating an iron-based alloy, but typically in fairly low proportions, such as the proportions of carbon, manganese, and others in various steels. Hence, high-entropy alloys are a novel class of materials. The term "high-entropy alloys" was coined by Taiwanese scientist Jien-Wei Yeh because the entropy increase of mixing is substantially higher when there is a larger number of elements in the mix, and their proportions are more nearly equal. Some alternative names, such as multi-component alloys, compositionally complex alloys and multi-principal-element alloys are also suggested by other researchers.

Brent Fultz is an American physicist and materials scientist and one of the world's leading authorities on statistical mechanics, diffraction, and phase transitions in materials. Fultz is the Barbara and Stanley Rawn, Jr. Professor of Applied Physics and Materials Science at the California Institute of Technology. He is known for his research in materials physics and materials chemistry, and for establishing the importance of phonon entropy to the phase stability of materials. Additionally, Fultz oversaw the construction of the wide angular-range chopper spectrometer (ARCS) instrument at the Spallation Neutron Source and has made advances in phonon measuring techniques.
Duplex stainless steels are a family of alloys with a two-phase microstructure consisting of both austenitic and ferritic phases. They offer excellent mechanical properties, corrosion resistance, and toughness compared to other types of stainless steel. However, duplex stainless steel can be susceptible to a phenomenon known as 475 °C embrittlement or duplex stainless steel age hardening, which is a type of aging process that causes loss of plasticity in duplex stainless steel when it is heated in the range of 250 °C (480 °F) to 550 °C (1,020 °F). At this temperature range, spontaneous phase separation of the ferrite phase into iron-rich and chromium-rich nanophases occurs, with no change in the mechanical properties of the austenite phase. This type of embrittlement is due to precipitation hardening, which makes the material become brittle and prone to cracking.











