Related Research Articles

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
Hot flashes are a form of flushing, often caused by the changing hormone levels that are characteristic of menopause. They are typically experienced as a feeling of intense heat with sweating and rapid heartbeat, and may typically last from two to 30 minutes for each occurrence.

The Women's Health Initiative (WHI) was a series of clinical studies initiated by the U.S. National Institutes of Health (NIH) in 1991, to address major health issues causing morbidity and mortality in postmenopausal women. It consisted of three clinical trials (CT) and an observational study (OS). In particular, randomized controlled trials were designed and funded that addressed cardiovascular disease, cancer, and osteoporosis.
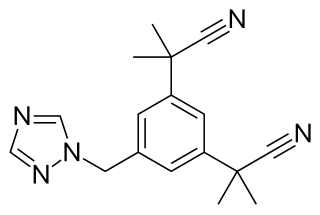
Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.

Raloxifene, sold under the brand name Evista among others, is a medication used to prevent and treat osteoporosis in postmenopausal women and those on glucocorticoids. For osteoporosis it is less preferred than bisphosphonates. It is also used to reduce the risk of breast cancer in those at high risk. It is taken by mouth.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.
Hypoestrogenism, or estrogen deficiency, refers to a lower than normal level of estrogen. It is an umbrella term used to describe estrogen deficiency in various conditions. Estrogen deficiency is also associated with an increased risk of cardiovascular disease, and has been linked to diseases like urinary tract infections and osteoporosis.

Polyestradiol phosphate (PEP), sold under the brand name Estradurin, is an estrogen medication which is used primarily in the treatment of prostate cancer in men. It is also used in women to treat breast cancer, as a component of hormone therapy to treat low estrogen levels and menopausal symptoms, and as a component of feminizing hormone therapy for transgender women. It is given by injection into muscle once every four weeks.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.
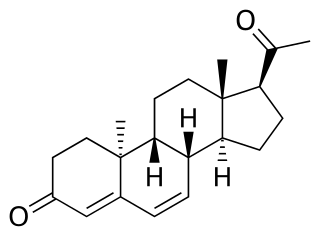
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Arzoxifene is a selective estrogen receptor modulator (SERM) of the benzothiophene group which was never marketed. It is a potent estrogen antagonist in mammary and uterine tissue while acting as an estrogen agonist to maintain bone density and lower serum cholesterol. Arzoxifene is a highly effective agent for prevention of mammary cancer induced in the rat by the carcinogen nitrosomethylurea and is significantly more potent than raloxifene in this regard. Arzoxifene is devoid of the uterotrophic effects of tamoxifen, suggesting that, in contrast to tamoxifen, it is unlikely that the clinical use of arzoxifene will increase the risk of developing endometrial carcinoma.
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. These symptoms can include hot flashes, vaginal atrophy, accelerated skin aging, vaginal dryness, decreased muscle mass, sexual dysfunction, and bone loss or osteoporosis. They are in large part related to the diminished levels of sex hormones that occur during menopause.
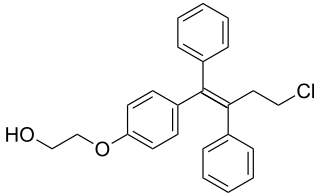
Ospemifene is an oral medication indicated for the treatment of dyspareunia – pain during sexual intercourse – encountered by some women, more often in those who are post-menopausal. Ospemifene is a selective estrogen receptor modulator (SERM) acting similarly to an estrogen on the vaginal epithelium, building vaginal wall thickness which in turn reduces the pain associated with dyspareunia. Dyspareunia is most commonly caused by "vulvar and vaginal atrophy."

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

8,9-Dehydroestrone, or Δ8-estrone, also known as estra-1,3,5(10),8-tetraen-3-ol-17-one, is a naturally occurring estrogen found in horses which is closely related to equilin, equilenin, and estrone, and, as the 3-sulfate ester sodium salt, is a minor constituent (3.5%) of conjugated estrogens (Premarin). It produces 8,9-dehydro-17β-estradiol as an important active metabolite, analogously to conversion of estrone or estrone sulfate into estradiol. The compound was first described in 1997. In addition to 8,9-dehydroestrone and 8,9-dehydro-17β-estradiol, 8,9-dehydro-17α-estradiol is likely also to be present in conjugated estrogens, but has not been identified at this time.

An estrogen (E) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy, and as part of feminizing hormone therapy for transgender women. They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of estrogens include bioidentical estradiol, natural conjugated estrogens, synthetic steroidal estrogens like ethinylestradiol, and synthetic nonsteroidal estrogens like diethylstilbestrol. Estrogens are one of three types of sex hormone agonists, the others being androgens/anabolic steroids like testosterone and progestogens like progesterone.

Estetrol (E4) is an estrogen medication and naturally occurring steroid hormone which is used in combination with a progestin in combined birth control pills and is under development for various other indications. These investigational uses include menopausal hormone therapy to treat symptoms such as vaginal atrophy, hot flashes, and bone loss and the treatment of breast cancer and prostate cancer. It is taken by mouth.
The Estrogen in Venous Thromboembolism Trial (EVTET) was a randomized controlled trial (RCT) of menopausal hormone therapy in 140 postmenopausal women with previous history of venous thromboembolism (VTE). It was a double-blind RCT of the estrogen, oral estradiol 2 mg/day, plus the progestogen, norethisterone acetate (NETA) (n=71) 1 mg/day versus placebo (n=69). The results of the trial were published in 2000 and 2001. The incidence of VTE was 10.7% in the hormone therapy group and 2.3% in the placebo group, with all events occurring within 261 days after study inclusion. The difference did not reach statistical significance in the sequential analysis, but was statistically significant if the sequential design was ignored. Markers of coagulation were likewise increased by hormone therapy. As a result of the high incidence of VTE in the treatment group, the trial was terminated prematurely. The researchers concluded on the basis of their findings that menopausal hormone therapy should not be used in women with a previous history of VTE.
The Menopause, Estrogen and Venous Events (MEVE) study was a retrospective observational study of menopausal hormone therapy and venous thromboembolism (VTE) in postmenopausal women with a previous history of VTE. It found that transdermal estradiol was not associated with increased risk of VTE whereas oral estrogens were associated with a large increase in risk. The mean dose of transdermal estradiol in the study was 50 μg/day, although data on dose were missing for around 50% of women. Similarly, a small study found that transdermal estradiol did not influence coagulation in women with prior VTE. These findings are similar to studies in menopausal women without prior history of VTE which have found that transdermal estradiol has minimal influence on coagulation and is not associated with increased risk of VTE at doses of up to 100 μg/day. Menopausal hormone therapy guidelines have cited the MEVE study and recommended use of transdermal estradiol over oral estrogens in women at high risk for VTE. However, randomized controlled trials (RCTs) are still needed to definitively confirm findings that transdermal estradiol is safer than oral estrogens in terms of VTE risk.
References
- ↑ Barrett-Connor E (December 1995). "Postmenopausal estrogen and heart disease". Atherosclerosis. 118 Suppl: S7–10. doi:10.1016/0021-9150(95)90068-3. PMID 8821460.
- ↑ Fitzpatrick LA, Good A (September 1999). "Micronized progesterone: clinical indications and comparison with current treatments". Fertil. Steril. 72 (3): 389–97. doi: 10.1016/s0015-0282(99)00272-1 . PMID 10519605.
- ↑ Stefanick ML (February 1999). "Estrogen, progestogens and cardiovascular risk". J Reprod Med. 44 (2 Suppl): 221–6. PMID 11392036.
- ↑ Pike MC (July 2005). "The role of mammographic density in evaluating changes in breast cancer risk". Gynecol. Endocrinol. 21 (Suppl 1): 1–5. doi:10.1080/09513590400030038. PMID 16112948. S2CID 39422465.