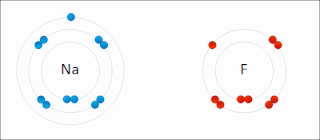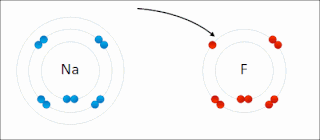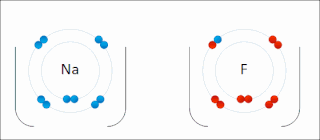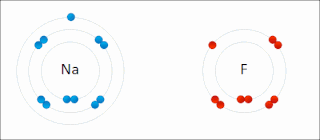
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. It is one of the main bonds along with Covalent bond and Metallic bonding. Ions are atoms that have gained one or more electrons and atoms that have lost one or more electrons. This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH+
4 or SO2−
4. In simpler words, an ionic bond is the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms.

Mass spectrometry (MS) is an analytical technique that ionizes chemical species and sorts the ions into a spectrum based on their mass-to-charge ratio. In simpler terms, a mass spectrum measures the masses within a sample. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.
In atomic, molecular, and solid-state physics, the electric field gradient (EFG) measures the rate of change of the electric field at an atomic nucleus generated by the electronic charge distribution and the other nuclei. The EFG couples with the nuclear electric quadrupole moment of quadrupolar nuclei to generate an effect which can be measured using several spectroscopic methods, such as nuclear magnetic resonance (NMR), microwave spectroscopy, electron paramagnetic resonance, nuclear quadrupole resonance (NQR), Mössbauer spectroscopy or perturbed angular correlation (PAC). The EFG is non-zero only if the charges surrounding the nucleus violate cubic symmetry and therefore generate an inhomogeneous electric field at the position of the nucleus.

Quadrupole magnets, abbreviated as Q-magnets, consist of groups of four magnets laid out so that in the planar multipole expansion of the field, the dipole terms cancel and where the lowest significant terms in the field equations are quadrupole. Quadrupole magnets are useful as they create a magnetic field whose magnitude grows rapidly with the radial distance from its longitudinal axis. This is used in particle beam focusing.

A Penning trap is a device for the storage of charged particles using a homogeneous axial magnetic field and an inhomogeneous quadrupole electric field. This kind of trap is particularly well suited to precision measurements of properties of ions and stable subatomic particles. Geonium atoms have been created and studied this way, to measure the electron magnetic moment. Recently these traps have been used in the physical realization of quantum computation and quantum information processing by trapping qubits. Penning traps are used in many laboratories worldwide, including CERN, to store antimatter like antiprotons.
Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance (NMR). Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectroscopy is referred to as "zero Field NMR." The NQR resonance is mediated by the interaction of the electric field gradient (EFG) with the quadrupole moment of the nuclear charge distribution. Unlike NMR, NQR is applicable only to solids and not liquids, because in liquids the quadrupole moment averages out. Because the EFG at the location of a nucleus in a given substance is determined primarily by the valence electrons involved in the particular bond with other nearby nuclei, the NQR frequency at which transitions occur is unique for a given substance. A particular NQR frequency in a compound or crystal is proportional to the product of the nuclear quadrupole moment, a property of the nucleus, and the EFG in the neighborhood of the nucleus. It is this product which is termed the nuclear quadrupole coupling constant for a given isotope in a material and can be found in tables of known NQR transitions. In NMR, an analogous but not identical phenomenon is the coupling constant, which is also the result of an internuclear interaction between nuclei in the analyte.
A quadrupole or quadrapole is one of a sequence of configurations of things like electric charge or current, or gravitational mass that can exist in ideal form, but it is usually just part of a multipole expansion of a more complex structure reflecting various orders of complexity.
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is mainly a magnetic dipole moment; the quadrupole moment does cause some small shifts in the hyperfine structure as well. All nuclei that have nonzero spin also possess a nonzero magnetic moment and vice versa, although the connection between the two quantities is not straightforward or easy to calculate.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. Similarly, biochemists use NMR to identify proteins and other complex molecules. Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules. The most common types of NMR are proton and carbon-13 NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin.

The quadrupole mass analyzer (QMS) is one type of mass analyzer used in mass spectrometry. It is also known as a transmission quadrupole mass spectrometer, quadrupole mass filter, or quadrupole mass spectrometer. As the name implies, it consists of four cylindrical rods, set parallel to each other. In a quadrupole mass spectrometer the quadrupole is the mass analyzer - the component of the instrument responsible for selecting sample ions based on their mass-to-charge ratio (m/z). Ions are separated in a quadrupole based on the stability of their trajectories in the oscillating electric fields that are applied to the rods.

The nuclear force is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience an electric force that tends to push them apart, but at short range the attractive nuclear force is strong enough to overcome the electromagnetic force. The nuclear force binds nucleons into atomic nuclei.
In an electron spectrometer, an incoming beam of electrons is bent with electric or magnetic fields. As higher energy electrons will be bent less by the beam, this produces a spatially distributed range of energies.
ISOLTRAP is a tandem Penning trap mass spectrometer at the On-Line Isotope Mass Separator at CERN. The facility plays a leading role in the field of high precision mass spectrometry of radioactive ions. The masses of more than 200 short-lived nuclides have been measured with a relative uncertainty of typically dm/m ~ 1x10−7 and even almost up to one order of magnitude lower in some special cases.

In accelerator physics strong focusing or alternating-gradient focusing is the principle that the net effect on a particle beam of charged particles passing through alternating field gradients is to make the beam converge. By contrast, weak focusing is the principle that nearby circles, described by charged particles moving in a uniform magnetic field, only intersect once per revolution.
Electric field NMR (EFNMR) spectroscopy differs from conventional NMR in that a sample containing suitable nuclei is polarised by a strong dc electric field instead of a constant magnetic field. The nuclei are stimulated (perturbed) by means of an alternating magnetic field, generated by passing an alternating current through a set of coils. The resulting magnetic resonance signal is small, and as in conventional NMR is typically sensed using a second set of coils and an amplifier.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong static magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics, crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Acoustic paramagnetic resonance (APR) is a phenomenon of resonant absorption of sound by a system of magnetic particles placed in an external magnetic field. It occurs when the energy of the sound wave quantum becomes equal to the splitting of the energy levels of the particles, the splitting being induced by the magnetic field. APR is a variation of electron paramagnetic resonance (EPR) where the acoustic rather than electromagnetic waves are absorbed by the studied sample. APR was theoretically predicted in 1952, independently by Semen Altshuler and Alfred Kastler, and was experimentally observed by W. G. Proctor and W. H. Tanttila in 1955.
Coulomb excitation is a technique in experimental nuclear physics to probe the electromagnetic aspect of nuclear structure. In coulomb excitation, a nucleus is excited by an inelastic collision with another nucleus through the electromagnetic interaction. In order to ensure that the interaction is electromagnetic in nature — and not nuclear — a "safe" scattering angle is chosen. This method is particularly useful for investigating collectivity in nuclei, as collective excitations are often connected by electric quadrupole transitions.











