
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.
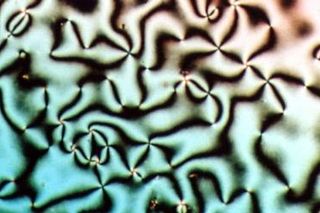
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties. The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter.

Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are injected from appropriate electrodes or are introduced by doping or photoexcitation.
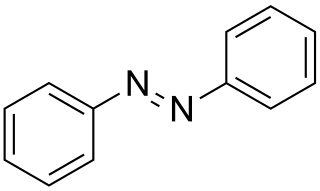
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a reversible process.

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another.

Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.
A photoswitch is a type of molecule that can change its structural geometry and chemical properties upon irradiation with electromagnetic radiation. Although often used interchangeably with the term molecular machine, a switch does not perform work upon a change in its shape whereas a machine does. However, photochromic compounds are the necessary building blocks for light driven molecular motors and machines. Upon irradiation with light, photoisomerization about double bonds in the molecule can lead to changes in the cis- or trans- configuration. These photochromic molecules are being considered for a range of applications.
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of intermolecular interactions. It is an interdisciplinary academic field, bridging solid-state and supramolecular chemistry.

3D optical data storage is any form of optical data storage in which information can be recorded or read with three-dimensional resolution.
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines.

Lyotropic liquid crystals result when amphiphiles, which are both hydrophobic and hydrophilic, dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water.
In chemistry, a halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bond, the result is not a formal chemical bond, but rather a strong electrostatic attraction. Mathematically, the interaction can be decomposed in two terms: one describing an electrostatic, orbital-mixing charge-transfer and another describing electron-cloud dispersion. Halogen bonds find application in supramolecular chemistry; drug design and biochemistry; crystal engineering and liquid crystals; and organic catalysis.
A high-refractive-index polymer (HRIP) is a polymer that has a refractive index greater than 1.50.
A liquid-crystal laser is a laser that uses a liquid crystal as the resonator cavity, allowing selection of emission wavelength and polarization from the active laser medium. The lasing medium is usually a dye doped into the liquid crystal. Liquid-crystal lasers are comparable in size to diode lasers, but provide the continuous wide spectrum tunability of dye lasers while maintaining a large coherence area. The tuning range is typically several tens of nanometers. Self-organization at micrometer scales reduces manufacturing complexity compared to using layered photonic metamaterials. Operation may be either in continuous wave mode or in pulsed mode.
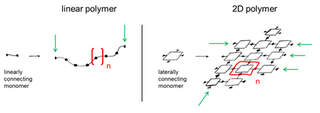
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.
A spiropyran is a type of organic chemical compound, known for photochromic properties that provide this molecule with the ability of being used in medical and technological areas. Spiropyrans were discovered in the early twentieth century. However, it was in the middle twenties when Fisher and Hirshbergin observed their photochromic characteristics and reversible reaction. In 1952, Fisher and co-workers announced for the first time photochromism in spiropyrans. Since then, there have been many studies on photochromic compounds that have continued up to the present.
Polymerization-induced phase separation (PIPS) is the occurrence of phase separation in a multicomponent mixture induced by the polymerization of one or more components. The increase in molecular weight of the reactive component renders one or more components to be mutually immiscible in one another, resulting in spontaneous phase segregation.











