Related Research Articles

Sunitinib is an oral, small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) on January 26, 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.

Lapatinib (INN), used in the form of lapatinib ditosylate (USAN) is an orally active drug for breast cancer and other solid tumours. It is a dual tyrosine kinase inhibitor which interrupts the HER2/neu and epidermal growth factor receptor (EGFR) pathways. It is used in combination therapy for HER2-positive breast cancer. It is used for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 (ErbB2).
Response evaluation criteria in solid tumors (RECIST) is a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatment. The criteria were published in February 2000 by an international collaboration including the European Organisation for Research and Treatment of Cancer (EORTC), National Cancer Institute of the United States, and the National Cancer Institute of Canada Clinical Trials Group. Today, the majority of clinical trials evaluating cancer treatments for objective response in solid tumors use RECIST. These criteria were developed and published in February 2000, and subsequently updated in 2009.
Progression-free survival (PFS) is "the length of time during and after the treatment of a disease, such as cancer, that a patient lives with the disease but it does not get worse". In oncology, PFS usually refers to situations in which a tumor is present, as demonstrated by laboratory testing, radiologic testing, or clinically. Similarly, "disease-free survival" is when patients have had operations and are left with no detectable disease.

Lenvatinib is an anti-cancer drug for the treatment of certain kinds of thyroid cancer, and potentially for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases.
Regorafenib is an oral multi-kinase inhibitor developed by Bayer which targets angiogenic, stromal and oncogenic receptor tyrosine kinase (RTK). Regorafenib shows anti-angiogenic activity due to its dual targeted VEGFR2-TIE2 tyrosine kinase inhibition. Since 2009 it was studied as a potential treatment option in multiple tumor types. By 2015 it had 2 US approvals for advanced cancers.
Avax Technologies, Inc is a Philadelphia based biotechnology company whose most advanced product candidate is MVax for melanoma. MVax is a cancer vaccine that received a Special Protocol Assessment agreement with the FDA in October 2006, and subsequently began a Phase III registration clinical trial in November 2007. In previous studies, MVax demonstrated a 5-year overall survival rate (OS)of 44% and response rate of 35%.
An imaging biomarker is a biologic feature, or biomarker detectable in an image. In medicine, an imaging biomarker is a feature of an image relevant to a patient's diagnosis. For example, a number of biomarkers are frequently used to determine risk of lung cancer. First, a simple lesion in the lung detected by X-ray, CT, or MRI can lead to the suspicion of a neoplasm. The lesion itself serves as a biomarker, but the minute details of the lesion serve as biomarkers as well, and can collectively be used to assess the risk of neoplasm. Some of the imaging biomarkers used in lung nodule assessment include size, spiculation, calcification, cavitation, location within the lung, rate of growth, and rate of metabolism. Each piece of information from the image represents a probability. Spiculation increases the probability of the lesion being cancer. A slow rate of growth indicates benignity. These variables can be added to the patient's history, physical exam, laboratory tests, and pathology to reach a proposed diagnosis. Imaging biomarkers can be measured using several techniques, such as CT, electroencephalography, magnetoencephalography, and MRI.

Brigatinib is a small-molecule targeted cancer therapy being developed by ARIAD Pharmaceuticals, Inc. Brigatinib acts as both a anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor.
Teprotumumab (RG-1507) is an experimental human monoclonal antibody developed by Genmab and Roche. It binds to IGF-1R.

Pembrolizumab is a humanized antibody used in cancer immunotherapy. It is an IgG4 isotype antibody that blocks a protective mechanism of cancer cells and thereby, allows the immune system to destroy them. It targets the programmed cell death protein 1 (PD-1) receptor of lymphocytes. The FDA initially approved it to treat metastatic melanoma. In 2017 the FDA approved it for any unresectable or metastatic solid tumor with certain genetic anomalies. This was the first time the FDA approved a cancer drug based on tumor genetics rather than tissue type or tumor site, therefore, Pembrolizumab is a so-called tissue-agnostic drug.
The United States Food and Drug Administration (FDA) initiated the FDA Accelerated Approval Program in 1992 to allow faster approval of drugs for serious conditions that fill an unmet medical need. The faster approval relies on use of surrogate endpoints. Drug approval typically requires clinical trials with endpoints that demonstrate a clinical benefit, such as increased survival for cancer patients. Drugs with accelerated approval can initially be tested in clinical trials that use a surrogate endpoint, or something that is thought to predict clinical benefit. Surrogate endpoints typically require less time, and in the case of a cancer patient, it is much faster to measure a reduction in tumor size, for example, than overall patient survival.

Idelalisib, sold under the brand name Zydelig, is a medication used to treat certain blood cancers.
An adaptive clinical trial is a clinical trial that evaluates a medical device or treatment by observing participant outcomes on a prescribed schedule, and modifying parameters of the trial protocol in accord with those observations. The adaptation process generally continues throughout the trial, as prescribed in the trial protocol. Modifications may include dosage, sample size, drug undergoing trial, patient selection criteria and "cocktail" mix. In some cases, trials have become an ongoing process that regularly adds and drops therapies and patient groups as more information is gained. Importantly, the trial protocol is set before the trial begins; the protocol pre-specifies the adaptation schedule and processes.
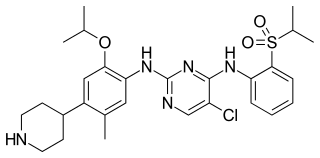
Ceritinib is a prescription-only drug used for the treatment non-small cell lung cancer (NSCLC). It was developed by Novartis and received FDA approval for use in April 2014.
Durvalumab is an FDA-approved immunotherapy for cancer, developed by Medimmune/AstraZeneca. It is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1) with the PD-1 and CD80 (B7.1) molecules. Durvalumab is approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who either have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
Atezolizumab is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).
Avelumab is a fully human monoclonal antibody developed by Merck KGaA and Pfizer as a pharmaceutical drug for use in immunotherapy, originally for the treatment of non-small-cell lung carcinoma (NSCLC).
Loncastuximab tesirine or ADCT-402 is an antibody-drug conjugate (ADC) composed of a humanized antibody targeting the protein CD19, which is expressed in a wide range of B cell hematological tumors. The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell non-Hodgkin lymphoma (NHL) and B-cell acute lymphoblastic leukemia (ALL).
References
- ↑ "NCI Dictionary of Cancer Terms". National Cancer Institute. Retrieved 5 June 2016.
- 1 2 Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics FDA
- ↑ Objective tumor response and RECIST criteria in cancer clinical trials
| This oncology article is a stub. You can help Wikipedia by expanding it. |