
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulas can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than are chemical names and structural formulas.

In chemistry, a ketone is a functional group with the structure RC(=O)R', where R and R' can be a variety of carbon-containing substituents. Ketones and aldehydes are simple compounds that contain a carbonyl group. They are considered "simple" because they do not have reactive groups like −OH or −Cl attached directly to the carbon atom in the carbonyl group, as in carboxylic acids containing −COOH. Many ketones are known and many are of great importance in industry and in biology. Examples include many sugars (ketoses) and the industrial solvent acetone, which is the smallest ketone.

In chemistry, an organic compound is generally any chemical compound that contains carbon. Due to carbon's ability to catenate, millions of organic compounds are known. Study of the properties and synthesis of organic compounds is the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds, along with a handful of other exceptions, are not classified as organic compounds and are considered inorganic. No consensus exists among chemists on precisely which carbon-containing compounds are excluded, making the definition of an organic compound elusive.

Organic chemistry is a subdiscipline of chemistry that studies the structure, properties and reactions of organic compounds, which contain carbon in covalent bonding. Study of structure determines their chemical composition and formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkaline, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and tin, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.

Eyes are organs of the visual system. They provide animals with vision, the ability to receive and process visual detail, as well as enabling several photo response functions that are independent of vision. Eyes detect light and convert it into electro-chemical impulses in neurons. In higher organisms, the eye is a complex optical system which collects light from the surrounding environment, regulates its intensity through a diaphragm, focuses it through an adjustable assembly of lenses to form an image, converts this image into a set of electrical signals, and transmits these signals to the brain through complex neural pathways that connect the eye via the optic nerve to the visual cortex and other areas of the brain. Eyes with resolving power have come in ten fundamentally different forms, and 96% of animal species possess a complex optical system. Image-resolving eyes are present in molluscs, chordates and arthropods.

In chemistry, an oxidizing agent is a substance that has the ability to oxidize other substances — in other words to cause them to lose electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

In linguistics, a compound verb or complex predicate is a multi-word compound that functions as a single verb. One component of the compound is a light verb or vector, which carries any inflections, indicating tense, mood, or aspect, but provides only fine shades of meaning. The other, "primary", component is a verb or noun which carries most of the semantics of the compound, and determines its arguments. It is usually in either base or [in Verb + Verb compounds] conjunctive participial form.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.
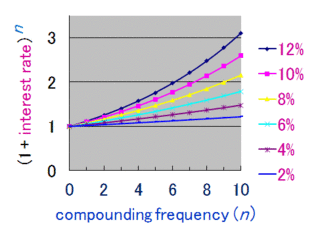
Compound interest is the addition of interest to the principal sum of a loan or deposit, or in other words, interest on interest. It is the result of reinvesting interest, rather than paying it out, so that interest in the next period is then earned on the principal sum plus previously accumulated interest. Compound interest is standard in finance and economics.
Halogenation is a chemical reaction that involves the addition of one or more halogens to a compound or material. The pathway and stoichiometry of halogenation depends on the structural features and functional groups of the organic substrate, as well as on the specific halogen. Inorganic compounds such as metals also undergo halogenation.
In linguistics, a compound is a lexeme that consists of more than one stem. Compounding, composition or nominal composition is the process of word formation that creates compound lexemes. That is, in familiar terms, compounding occurs when two or more words or signs are joined to make one longer word or sign. The meaning of the compound may be similar to or different from the meaning of its components in isolation. The component stems of a compound may be of the same part of speech—as in the case of the English word footpath, composed of the two nouns foot and path—or they may belong to different parts of speech, as in the case of the English word blackbird, composed of the adjective black and the noun bird. With very few exceptions, English compound words are stressed on their first component stem.

Pyrazolopyrimidines are a series of isomeric heterocyclic chemical compounds with the molecular formula C6H5N3. They form the central core of a variety of more complex chemical compounds including some pharmaceuticals and pesticides.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic, in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations.

A chemical compound is a chemical substance composed of many identical molecules composed of atoms from more than one element held together by chemical bonds. A chemical element bonded to an identical chemical element is not a chemical compound since only one element, not two different elements, is involved.

Baron Jöns Jacob Berzelius, known throughout his life as Jacob Berzelius, was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be one of the founders of modern chemistry.














