Readings
- Aickin M. (2004) "Separation Tests for Early-Phase Complementary and Alternative Medicine Comparative Trials". Evidence-Based Integrative Medicine, 1(4), 225–231
A separation test is a statistical procedure for early-phase research, to decide whether to pursue further research. It is designed to avoid the prevalent situation in early-phase research, when a statistically underpowered test gives a negative result.

Neurology is the branch of medicine dealing with the diagnosis and treatment of all categories of conditions and disease involving the nervous system, which comprises the brain, the spinal cord and the peripheral nerves. Neurological practice relies heavily on the field of neuroscience, the scientific study of the nervous system.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc., which have been dissolved into liquid solutions.

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Proof of concept, also known as proof of principle, is a realization of a certain idea, method or principle in order to demonstrate its feasibility, or viability, or a demonstration in principle with the aim of verifying that some concept or theory has practical potential. A proof of concept is usually small and may or may not be complete.

A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed.
Margaret Schönberger Mahler was an Austrian-American psychiatrist, psychoanalyst, and pediatrician. She did pioneering work in the field of infant and young child research. On the basis of empirical studies, she developed a development model that became particularly influential in psychoanalysis and Object relations theory. Mahler developed the separation–individuation theory of child development.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.
In statistics, sequential analysis or sequential hypothesis testing is statistical analysis where the sample size is not fixed in advance. Instead data is evaluated as it is collected, and further sampling is stopped in accordance with a pre-defined stopping rule as soon as significant results are observed. Thus a conclusion may sometimes be reached at a much earlier stage than would be possible with more classical hypothesis testing or estimation, at consequently lower financial and/or human cost.
Theralizumab is an immunomodulatory drug developed by Thomas Hünig of the University of Würzburg. It was withdrawn from development after inducing severe inflammatory reactions as well as chronic organ failure in the first-in-human study by Parexel in London in March 2006. The developing company, TeGenero Immuno Therapeutics, went bankrupt later that year. The commercial rights were then acquired by a Russian startup, TheraMAB. The drug was renamed TAB08. Phase I and II clinical trials have been completed for arthritis and clinical trials have been initiated for cancer.
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness (efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. These research procedures are designed for the prevention, treatment, diagnosis or understanding of disease symptoms.
Medical statistics deals with applications of statistics to medicine and the health sciences, including epidemiology, public health, forensic medicine, and clinical research. Medical statistics has been a recognized branch of statistics in the United Kingdom for more than 40 years, but the term has not come into general use in North America, where the wider term 'biostatistics' is more commonly used. However, "biostatistics" more commonly connotes all applications of statistics to biology. Medical statistics is a subdiscipline of statistics.
It is the science of summarizing, collecting, presenting and interpreting data in medical practice, and using them to estimate the magnitude of associations and test hypotheses. It has a central role in medical investigations. It not only provides a way of organizing information on a wider and more formal basis than relying on the exchange of anecdotes and personal experience, but also takes into account the intrinsic variation inherent in most biological processes.
The Thalmann Algorithm is a deterministic decompression model originally designed in 1980 to produce a decompression schedule for divers using the US Navy Mk15 rebreather. It was developed by Capt. Edward D. Thalmann, MD, USN, who did research into decompression theory at the Naval Medical Research Institute, Navy Experimental Diving Unit, State University of New York at Buffalo, and Duke University. The algorithm forms the basis for the current US Navy mixed gas and standard air dive tables. The decompression model is also referred to as the Linear–Exponential model or the Exponential–Linear model.
A glossary of terms used in clinical research.
Tanezumab is a monoclonal antibody against nerve growth factor as a treatment for pain via a novel mechanisms different from conventional pain-killer drugs. Tanezumab was discovered and developed by Rinat Neuroscience and was acquired by Pfizer in 2006.
Cytel is a multinational statistical software developer and contract research organization, headquartered in Cambridge, Massachusetts, USA. Cytel provides clinical trial design, implementation services, and statistical products primarily for the biotech and pharmaceutical development markets.

The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.
Separation anxiety disorder (SAD) is an anxiety disorder in which an individual experiences excessive anxiety regarding separation from home and/or from people to whom the individual has a strong emotional attachment. Separation anxiety is a natural part of the developmental process. It is most common in infants and little children, typically between the ages of six to seven months to three years, although it may pathologically manifest itself in older children, adolescents and adults. Unlike SAD, normal separation anxiety indicates healthy advancements in a child's cognitive maturation and should not be considered a developing behavioral problem.

Zicronapine is an atypical antipsychotic medication formerly under development by H. Lundbeck A/S. In phase II studies zicronapine showed statistically significant separation from placebo and convincing efficacy and safety data when compared to olanzapine.

The thermodynamic model was one of the first decompression models in which decompression is controlled by the volume of gas bubbles coming out of solution. In this model, pain only DCS is modelled by a single tissue which is diffusion-limited for gas uptake and bubble-formation during decompression causes "phase equilibration" of partial pressures between dissolved and free gases. The driving mechanism for gas elimination in this tissue is inherent unsaturation, also called partial pressure vacancy or the oxygen window, where oxygen metabolised is replaced by more soluble carbon dioxide. This model was used to explain the effectiveness of the Torres Straits Island pearl divers empirically developed decompression schedules, which used deeper decompression stops and less overall decompression time than the current naval decompression schedules. This trend to deeper decompression stops has become a feature of more recent decompression models.
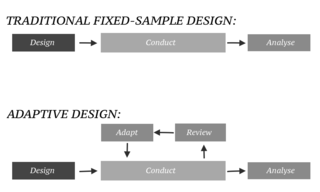
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.