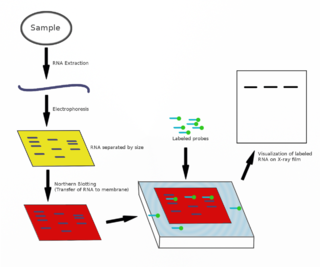
The northern blot, or RNA blot, is a technique used in molecular biology research to study gene expression by detection of RNA in a sample.
Immunoperoxidase is a type of immunostain used in molecular biology, medical research, and clinical diagnostics. In particular, immunoperoxidase reactions refer to a sub-class of immunohistochemical or immunocytochemical procedures in which the antibodies are visualized via a peroxidase-catalyzed reaction.

A DNA microarray is a collection of microscopic DNA spots attached to a solid surface. Scientists use DNA microarrays to measure the expression levels of large numbers of genes simultaneously or to genotype multiple regions of a genome. Each DNA spot contains picomoles of a specific DNA sequence, known as probes. These can be a short section of a gene or other DNA element that are used to hybridize a cDNA or cRNA sample under high-stringency conditions. Probe-target hybridization is usually detected and quantified by detection of fluorophore-, silver-, or chemiluminescence-labeled targets to determine relative abundance of nucleic acid sequences in the target. The original nucleic acid arrays were macro arrays approximately 9 cm × 12 cm and the first computerized image based analysis was published in 1981. It was invented by Patrick O. Brown. An example of its application is in SNPs arrays for polymorphisms in cardiovascular diseases, cancer, pathogens and GWAS analysis. It is also used for the identification of structural variations and the measurement of gene expression.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.

In molecular biology, biochips are engineered substrates that can host large numbers of simultaneous biochemical reactions. One of the goals of biochip technology is to efficiently screen large numbers of biological analytes, with potential applications ranging from disease diagnosis to detection of bioterrorism agents. For example, digital microfluidic biochips are under investigation for applications in biomedical fields. In a digital microfluidic biochip, a group of (adjacent) cells in the microfluidic array can be configured to work as storage, functional operations, as well as for transporting fluid droplets dynamically.
Plate readers, also known as microplate readers or microplate photometers, are instruments which are used to detect biological, chemical or physical events of samples in microtiter plates. They are widely used in research, drug discovery, bioassay validation, quality control and manufacturing processes in the pharmaceutical and biotechnological industry and academic organizations. Sample reactions can be assayed in 1-1536 well format microtiter plates. The most common microplate format used in academic research laboratories or clinical diagnostic laboratories is 96-well with a typical reaction volume between 100 and 200 µL per well. Higher density microplates are typically used for screening applications, when throughput and assay cost per sample become critical parameters, with a typical assay volume between 5 and 50 µL per well. Common detection modes for microplate assays are absorbance, fluorescence intensity, luminescence, time-resolved fluorescence, and fluorescence polarization.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.
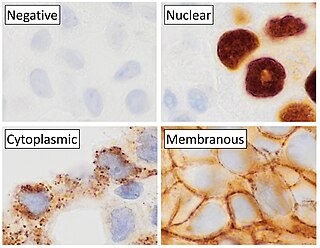
Immunohistochemistry (IHC) is a form of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells and tissue, by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Albert Hewett Coons, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941. This led to the later development of immunohistochemistry.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
A protein microarray is a high-throughput method used to track the interactions and activities of proteins, and to determine their function, and determining function on a large scale. Its main advantage lies in the fact that large numbers of proteins can be tracked in parallel. The chip consists of a support surface such as a glass slide, nitrocellulose membrane, bead, or microtitre plate, to which an array of capture proteins is bound. Probe molecules, typically labeled with a fluorescent dye, are added to the array. Any reaction between the probe and the immobilised protein emits a fluorescent signal that is read by a laser scanner. Protein microarrays are rapid, automated, economical, and highly sensitive, consuming small quantities of samples and reagents. The concept and methodology of protein microarrays was first introduced and illustrated in antibody microarrays in 1983 in a scientific publication and a series of patents. The high-throughput technology behind the protein microarray was relatively easy to develop since it is based on the technology developed for DNA microarrays, which have become the most widely used microarrays.
The detection of genetically modified organisms in food or feed is possible by biochemical means. It can either be qualitative, showing which genetically modified organism (GMO) is present, or quantitative, measuring in which amount a certain GMO is present. Being able to detect a GMO is an important part of GMO labeling, as without detection methods the traceability of GMOs would rely solely on documentation.
Loop-mediated isothermal amplification (LAMP) is a single-tube technique for the amplification of DNA and a low-cost alternative to detect certain diseases that was invented in 2000 at the University of Tokyo. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) combines LAMP with a reverse transcription step to allow the detection of RNA.
A nitrocellulose slide is a glass microscope slide that is coated with nitrocellulose that is used to bind biological material, often protein, for colorimetric and fluorescence detection assays. For this purpose, a nitrocellulose slide is generally considered to be superior to glass, because it binds a great deal more protein, and protects the tertiary structure of the protein. Typically, nitrocellulose slides have a thin, opaque film of nitrocellulose on a standard 25mm × 75 mm glass microscope slide. The film is extremely sensitive to contact, and to foreign material; contact causes deformation and deposition of material, especially liquids.
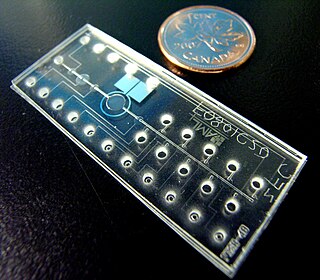
Bio-MEMS is an abbreviation for biomedical microelectromechanical systems. Bio-MEMS have considerable overlap, and is sometimes considered synonymous, with lab-on-a-chip (LOC) and micro total analysis systems (μTAS). Bio-MEMS is typically more focused on mechanical parts and microfabrication technologies made suitable for biological applications. On the other hand, lab-on-a-chip is concerned with miniaturization and integration of laboratory processes and experiments into single chips. In this definition, lab-on-a-chip devices do not strictly have biological applications, although most do or are amenable to be adapted for biological purposes. Similarly, micro total analysis systems may not have biological applications in mind, and are usually dedicated to chemical analysis. A broad definition for bio-MEMS can be used to refer to the science and technology of operating at the microscale for biological and biomedical applications, which may or may not include any electronic or mechanical functions. The interdisciplinary nature of bio-MEMS combines material sciences, clinical sciences, medicine, surgery, electrical engineering, mechanical engineering, optical engineering, chemical engineering, and biomedical engineering. Some of its major applications include genomics, proteomics, molecular diagnostics, point-of-care diagnostics, tissue engineering, single cell analysis and implantable microdevices.
A reverse phase protein lysate microarray (RPMA) is a protein microarray designed as a dot-blot platform that allows measurement of protein expression levels in a large number of biological samples simultaneously in a quantitative manner when high-quality antibodies are available.

MAGIChips, also known as "microarrays of gel-immobilized compounds on a chip" or "three-dimensional DNA microarrays", are devices for molecular hybridization produced by immobilizing oligonucleotides, DNA, enzymes, antibodies, and other compounds on a photopolymerized micromatrix of polyacrylamide gel pads of 100x100x20µm or smaller size. This technology is used for analysis of nucleic acid hybridization, specific binding of DNA, and low-molecular weight compounds with proteins, and protein-protein interactions.
Chromogenic in situ hybridization (CISH) is a cytogenetic technique that combines the chromogenic signal detection method of immunohistochemistry (IHC) techniques with in situ hybridization. It was developed around the year 2000 as an alternative to fluorescence in situ hybridization (FISH) for detection of HER-2/neu oncogene amplification. CISH is similar to FISH in that they are both in situ hybridization techniques used to detect the presence or absence of specific regions of DNA. However, CISH is much more practical in diagnostic laboratories because it uses bright-field microscopes rather than the more expensive and complicated fluorescence microscopes used in FISH.

The centrifugal micro-fluidic biochip or centrifugal micro-fluidic biodisk is a type of lab-on-a-chip technology, also known as lab-on-a-disc, that can be used to integrate processes such as separating, mixing, reaction and detecting molecules of nano-size in a single piece of platform, including a compact disk or DVD. This type of micro-fluidic biochip is based upon the principle of microfluidics; to take advantage of non-inertial pumping for lab-on-a-chip devices using non-inertial valves and switches under centrifugal force and Coriolis effect to distribute fluids about the disks in a highly parallel order.

A peptide microarray is a collection of peptides displayed on a solid surface, usually a glass or plastic chip. Peptide chips are used by scientists in biology, medicine and pharmacology to study binding properties and functionality and kinetics of protein-protein interactions in general. In basic research, peptide microarrays are often used to profile an enzyme, to map an antibody epitope or to find key residues for protein binding. Practical applications are seromarker discovery, profiling of changing humoral immune responses of individual patients during disease progression, monitoring of therapeutic interventions, patient stratification and development of diagnostic tools and vaccines.
Ampliphox is a colorimetric detection technology created as a research tool for the analysis of low-density microarrays using colorimetric detection. It combines a reagent kit and a benchtop instrument for detection for low-density microarray applications and is produced by InDevR, Inc. The ampliphox provides equivalent analytical sensitivity to fluorescence by using colorimetric readout.
Alexandre I et al. Anal Biochem. 2001 Aug 1;295(1):1-8.









