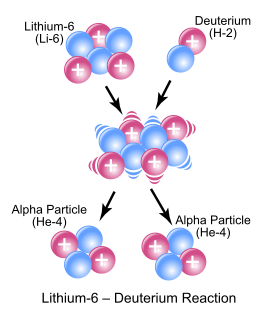Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of +2 e and a mass of 4 u. For example, uranium-238 decays to form thorium-234. Alpha particles have a charge +2 e, but as a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms – the charge is not usually shown.

In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta ray is emitted from an atomic nucleus. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino, or conversely a proton is converted into a neutron by the emission of a positron with a neutrino, thus changing the nuclide type. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energetically possible, the energy release or Q value must be positive.
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources.

A Geiger counter is an instrument used for detecting and measuring ionizing radiation. Also known as a Geiger-Mueller counter, it is widely used in applications such as radiation dosimetry, radiological protection, experimental physics, and the nuclear industry.

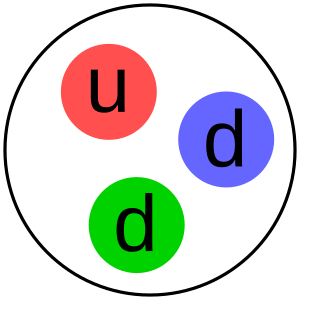
The neutron is a subatomic particle, symbol
n
or
n0
, with no net electric charge and a mass slightly larger than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics.
Particle radiation is the radiation of energy by means of fast-moving subatomic particles. Particle radiation is referred to as a particle beam if the particles are all moving in the same direction, similar to a light beam.

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:

A beta particle, also called beta ray or beta radiation, is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively.

Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation, such as an alpha particle, beta particle with neutrino or only a neutrino in the case of electron capture, or a gamma ray or electron in the case of internal conversion. A material containing such unstable nuclei is considered radioactive. Certain highly excited short-lived nuclear states can decay through neutron emission, or more rarely, proton emission.

A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the excitation effect of incident radiation on a scintillating material, and detecting the resultant light pulses.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". The IAEA also states "The accepted understanding of the term radiation protection is restricted to protection of people. Suggestions to extend the definition to include the protection of non-human species or the protection of the environment are controversial". Exposure can be from a radiation source external to the human body or due to the bodily intake of a radioactive material.
Neutron radiation is a form of ionizing radiation that presents as free neutrons. Typical phenomena are nuclear fission or nuclear fusion causing the release of free neutrons, which then react with nuclei of other atoms to form new isotopes—which, in turn, may trigger further neutron radiation. Free neutrons are unstable, decaying into a proton, an electron, plus an anti-electron-neutrino with a mean lifetime of 887 seconds.
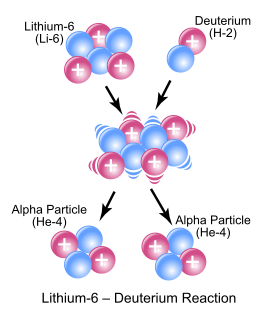
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce one or more nuclides that are different from the nuclide(s) that began the process. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction.

An air shower is an extensive cascade of ionized particles and electromagnetic radiation produced in the atmosphere when a primary cosmic ray enters the atmosphere. When a particle, which could be a proton, a nucleus, an electron, a photon, or (rarely) a positron, strikes an atom's nucleus in the air it produces many energetic hadrons. The unstable hadrons decay in the air speedily into other particles and electromagnetic radiation, which are part of the shower components. The secondary radiation rains down, including x-rays, muons, protons, antiprotons, alpha particles, pions, electrons, positrons, and neutrons.

In dosimetry, linear energy transfer (LET) is the amount of energy that an ionizing particle transfers to the material traversed per unit distance. It describes the action of radiation into matter.
Naturally Occurring Radioactive Materials (NORM) and Technologically Enhanced Naturally Occurring Radioactive Materials (TENORM) consist of materials, usually industrial wastes or by-products enriched with radioactive elements found in the environment, such as uranium, thorium and potassium and any of their decay products, such as radium and radon.
Radiobiology is a field of clinical and basic medical sciences that involves the study of the action of ionizing radiation on living things, especially health effects of radiation. Ionizing radiation is generally harmful and potentially lethal to living things but can have health benefits in radiation therapy for the treatment of cancer and thyrotoxicosis. Its most common impact is the induction of cancer with a latent period of years or decades after exposure. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Controlled doses are used for medical imaging and radiotherapy.
In radiobiology, the relative biological effectiveness is the ratio of biological effectiveness of one type of ionizing radiation relative to another, given the same amount of absorbed energy. The RBE is an empirical value that varies depending on the particles, energies involved, and which biological effects are deemed relevant.

A gamma ray or gamma radiation, is a penetrating electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves and so imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; he had previously discovered two less penetrating types of decay radiation, which he named alpha rays and beta rays in ascending order of penetrating power.

Alpha particles, also called alpha ray or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+
or 4
2He2+
indicating a helium ion with a +2 charge. If the ion gains electrons from its environment, the alpha particle becomes a normal helium atom 4
2He.