Tetrachlorodifluoroethane is the name for two isomers.
- Tetrachloro-1,2-difluoroethane, also known as CFC-112
- Tetrachloro-1,1-difluoroethane, also known as CFC-112a
Tetrachlorodifluoroethane is the name for two isomers.

The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force on 1 January 1989. Since then, it has undergone nine revisions, in 1990 (London), 1991 (Nairobi), 1992 (Copenhagen), 1993 (Bangkok), 1995 (Vienna), 1997 (Montreal), 1998 (Australia), 1999 (Beijing) and 2016 (Kigali) As a result of the international agreement, the ozone hole in Antarctica is slowly recovering. Climate projections indicate that the ozone layer will return to 1980 levels between 2040 and 2066. Due to its widespread adoption and implementation, it has been hailed as an example of successful international co-operation. Former UN Secretary-General Kofi Annan stated that "perhaps the single most successful international agreement to date has been the Montreal Protocol". In comparison, effective burden-sharing and solution proposals mitigating regional conflicts of interest have been among the success factors for the ozone depletion challenge, where global regulation based on the Kyoto Protocol has failed to do so. In this case of the ozone depletion challenge, there was global regulation already being installed before a scientific consensus was established. Also, overall public opinion was convinced of possible imminent risks.

Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.

A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.

Gawad Kalinga (GK) ("to give care" in Tagalog) is a Philippine poverty alleviation and nation-building movement known officially as the Gawad Kalinga Community Development Foundation.
Dichlorodifluoromethane (R-12) is a colorless gas usually sold under the brand name Freon-12, and a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was banned in developed countries in 1996, and in developing countries in 2010 out of concerns about its damaging effect on the ozone layer. Its only allowed usage is as a fire retardant in submarines and aircraft. It is soluble in many organic solvents. R-12 cylinders are colored white.
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer.
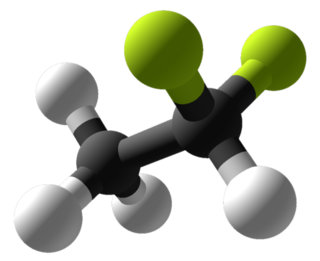
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula C2H4F2. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential (124) and a shorter atmospheric lifetime (1.4 years).
Dichloroethane can refer to either of two isomeric organochlorides with the molecular formula C2H4Cl2:
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive, nontoxic chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, and long atmospheric lifetime. Only a few other greenhouse gases surpass CFC-13 in global warming potential (GWP). The IPCC AR5 reported that CFC-13's Atmospheric lifetime was 640 years.

In the study of conformational isomerism, the Gauche effect is an atypical situation where a gauche conformation is more stable than the anti conformation (180°).
The molecular formula C2H4F2 (molar mass: 66.05 g/mol, exact mass: 66.0281 u) may refer to:
Difluoroethane may refer to:

1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.
Difluoroethylene may refer to:
Difluoroethene or Difluoroethylene can refer to any one of several isomeric forms of the organochloride with the molecular formula C2H2F2:
Stanbic Holdings Plc, formerly known as CfC Stanbic Holdings Limited, is a financial services organization in Kenya. The Group's headquarters are located in Nairobi, Kenya, with subsidiaries in Kenya and South Sudan. Stanbic Holdings is a member of the Standard Bank Group, a financial services giant based in South Africa. The institution is licensed and governed by the Central Bank of Kenya, the national banking regulator.
1,2-Difluoroethane is a saturated hydrofluorocarbon containing an atom of fluorine attached to each of two carbons atoms. The formula can be written CH2FCH2F. It is an isomer of 1,1-difluoroethane. It has a HFC name of HFC-152 with no letter suffix. When cooled to cryogenic temperatures it can have different conformers, gauche and trans. In the liquid form these are about equally abundant and easily interconvert. As a gas it is mostly the gauche form.

Tetrachloro-1,1-difluoroethane or 1,1,1,2-tetrachloro-2,2-difluoroethane, Freon 112a, R-112a, or CFC-112a is an asymmetric chlorofluorocarbon isomer of tetrachloro-1,1-difluoroethane with formula CClF2CCl3. It contains ethane substituted by four chlorine atoms and two fluorine atoms. With a boiling point of 91.5° it is the freon with second highest boiling point.

Tetrachloro-1,2-difluoroethane is a chlorofluorocarbon known as Freon 112, CFC-112 or R-112. It has a symmetrical structure CCl2FCCl2F and so can be called symmetrical tetrachlorodifluoroethane. "Symmetrical" may also be abbreviated to "s-" or "sym-". In contrast an asymmetrical isomer has formula CCl3CClF2.