In chemistry, the law of multiple proportions states that if two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole numbers. This law is sometimes called Dalton's Law, named after John Dalton, the chemist who first expressed it.

Tin is a chemical element with the symbol Sn (from Latin: stannum) and atomic number 50. Tin is a silvery metal that characteristically has a faint yellow hue. Tin, like indium, is soft enough to be cut without much force. When a bar of tin is bent, the so-called “tin cry” can be heard as a result of sliding tin crystals reforming; this trait is shared by indium, cadmium, and frozen mercury. Pure tin after solidifying keeps a mirror-like appearance similar to most metals. However, in most tin alloys (such as pewter), the metal solidifies with a dull gray color. Tin is a post-transition metal in group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, SnO2. Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element on Earth and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table, thanks to its magic number of protons. It has two main allotropes: at room temperature, the stable allotrope is β-tin, a silvery-white, malleable metal, but at low temperatures, it transforms into the less dense grey α-tin, which has the diamond cubic structure. Metallic tin does not easily oxidize in air.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide ((C2H5)2SnI2), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn-C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Tin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating. Tin(II) chloride should not be confused with the other chloride of tin; tin(IV) chloride or stannic chloride (SnCl4).

Tin(II) oxide is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.
Octahedral clusters are inorganic or organometallic cluster compounds composed of six metals in an octahedral array. Many types of compounds are known, but all are synthetic.

Tin(II) hydroxide, Sn(OH)2, also known as stannous hydroxide, is an inorganic compound tin(II). The only related material for which definitive information is available is the oxy hydroxide Sn6O4(OH)4, but other related materials are claimed. They are all white solids that are insoluble in water.
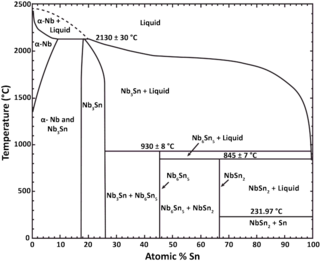
Niobium–tin is an intermetallic compound of niobium (Nb) and tin (Sn), used industrially as a type II superconductor. This intermetallic compound has a simple structure: A3B. It is more expensive than niobium-titanium (NbTi), but remains superconducting up to a magnetic flux density of 30 teslas [T] (300,000 G), compared to a limit of roughly 15 T for NbTi.

Tin-glazing is the process of giving tin-glazed pottery items a ceramic glaze that is white, glossy and opaque, which is normally applied to red or buff earthenware. Tin-glaze is plain lead glaze with a small amount of tin oxide added. The opacity and whiteness of tin glaze encourage its frequent decoration. Historically this has mostly been done before the single firing, when the colours blend into the glaze, but since the 17th century also using overglaze enamels, with a light second firing, allowing a wider range of colours. Majolica, maiolica, delftware and faience are among the terms used for common types of tin-glazed pottery.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.

Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin stannum, 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.

Cerium(III) oxide, also known as cerium oxide, cerium trioxide, cerium sesquioxide, cerous oxide or dicerium trioxide, is an oxide of the rare-earth metal cerium. It has chemical formula Ce2O3 and is gold-yellow in color.

Sodium stannate, formally sodium hexahydroxostannate(IV), is the inorganic compound with the formula Na2[Sn(OH)6]. This colourless salt forms upon dissolving metallic tin or tin(IV) oxide in sodium hydroxide, and is used as a stabiliser for hydrogen peroxide. In older literature, stannates are sometimes represented as having the simple oxyanion SnO32−, in which case this compound is sometimes named as sodium stannate–3–water and represented as Na2SnO3·3H2O, a hydrate with three waters of crystallisation. The anhydrous form of sodium stannate, Na2SnO3, is recognised as a distinct compound with its own CAS Registry Number, 12058-66-1, and a distinct material safety data sheet.

Ixiolite is an accessory oxide mineral found in granitic pegmatites. It is an oxide with the general chemical formula (Ta,Nb,Sn,Mn,Fe)4O8 or (Ta,Mn,Nb)O2.

Lead-tin-yellow is a yellow pigment, of historical importance in oil painting, sometimes called the "Yellow of the Old Masters" because of the frequency with which it was used by those famous painters.














