Other uses
- TRIS online, a former bibliographic database created by U.S. Department of Transportation Research Information Services online
- TRIS, the ICAO type designator for the Britten-Norman Trislander
Tris or TRIS may refer to:
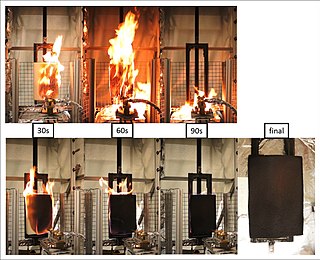
The term flame retardant subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and prevent or slow the further development of flames by a variety of different physical and chemical mechanisms. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive, while organohalogen and organophosphorus compounds can be either reactive or additive.
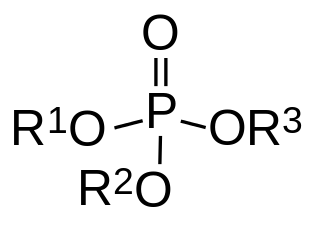
In organic chemistry, organophosphates (also known as phosphate esters, phosphorates(V) or OPEs) are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides.
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric acid with n-butanol.
Brominated flame retardants (BFRs) are organobromine compounds that have an inhibitory effect on combustion chemistry and tend to reduce the flammability of products containing them. The brominated variety of commercialized chemical flame retardants comprise approximately 19.7% of the market. They are effective in plastics and textile applications like electronics, clothes, and furniture.

A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are also available as coatings or sprays to be applied to an object.

Ammonium sulfamate is a white crystalline solid, readily soluble in water. It is commonly used as a broad spectrum herbicide, with additional uses as a compost accelerator, flame retardant and in industrial processes.

Tricresyl phosphate (TCP), is a mixture of three isomeric organophosphate compounds most notably used as a flame retardant. Other uses include as a plasticizer in manufacturing for lacquers and varnishes and vinyl plastics and as an antiwear additive in lubricants. Pure tricresyl phosphate is a colourless, viscous liquid, although commercial samples are typically yellow. It is virtually insoluble in water, but easily soluble in organic solvents like toluene, hexane, and diethylether among others. It was synthesized by Alexander Williamson in 1854 upon reacting phosphorus pentachloride with cresol, though today's manufacturers can prepare TCP by mixing cresol with phosphorus oxychloride or phosphoric acid as well. TCP, especially the all-ortho isomer, is the causative agent in a number of acute poisonings. Its chronic toxicity is also of concern. The ortho-isomer is rarely used on its own outside of laboratory studies that require isomeric purity, due to its extremely toxic nature, and is generally excluded from commercial products where TCP is involved.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).

Triphenyl phosphate (TPhP) is the chemical compound with the formula OP(OC6H5)3. It is the simplest aromatic organophosphate. This colourless solid is the ester (triester) of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant in a wide variety of settings and products.
PFR may refer to:

Ammonium polyphosphate is an inorganic salt of polyphosphoric acid and ammonia containing both chains and possibly branching. Its chemical formula is H(NH4PO3)nOH showing that each monomer consists of an orthophosphate radical of a phosphorus atom with three oxygens and one negative charge neutralized by an ammonium cation leaving two bonds free to polymerize. In the branched cases some monomers are missing the ammonium anion and instead link to three other monomers.

Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is an organophosphorus compound with the chemical formula [P(CH2OH)4]Cl. The cation P(CH2OH)4+ is four-coordinate, as is typical for phosphonium salts. THPC has applications as a precursor to fire-retardant materials, as well as a microbiocide in commercial and industrial water systems.

Tris(1,3-dichloroisopropyl)phosphate (TDCPP) is a chlorinated organophosphate. Organophosphate chemicals have a wide variety of applications and are used as flame retardants, pesticides, plasticizers, and nerve gases. TDCPP is structurally similar to several other organophosphate flame retardants, such as tris(2-chloroethyl) phosphate (TCEP) and tris(chloropropyl)phosphate (TCPP). TDCPP and these other chlorinated organophosphate flame retardants are all sometimes referred to as "chlorinated tris".

Tris(2,3-dibromopropyl) phosphate ("tris") is a chemical once widely used as a flame retardant in plastics and textiles.
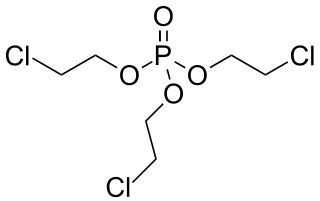
Tris(2-chloroethyl) phosphate (TCEP) is a chemical compound used as a flame retardant, plasticizer, and viscosity regulator in various types of polymers including polyurethanes, polyester resins, and polyacrylates.
Silent Spring Institute is a nonprofit organization dedicated to studying and reporting primarily on breast cancer prevention, although its research covers other health-related topics as well.

2-Ethylhexyl diphenyl phosphate (Octicizer) is an organophosphate compound. It acts as both a plasticizer and flame retardant in PVC, its wide liquid range also makes it suitable as a flame retardant in hydraulic fluids. It has low acute toxicity in feeding experiments, but has been implicated as a potential hormone mimetic.

Tris(2-ethylhexyl)phosphate (TEHP) is an organic chemical compound in the organophosphate group. It is a triakylphosphate.
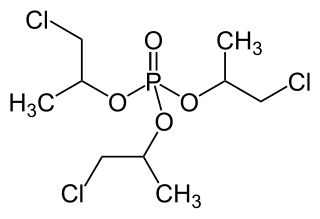
Tris(chloropropyl) phosphate is a chlorinated organophosphate flame retardant commonly added to polyurethane foams. Comparatively minor amounts are used in PVC and EVA.

Trixylyl phosphate (TXP) is an aromatic phosphate ester. It was historically used as a flame retardant for acetate plastics and PVC. It also saw significant use as a fire-resistant hydraulic fluid.