
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity. When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.

A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants. In a PWR, the primary coolant (water) is pumped under high pressure to the reactor core where it is heated by the energy released by the fission of atoms. The heated, high pressure water then flows to a steam generator, where it transfers its thermal energy to lower pressure water of a secondary system where steam is generated. The steam then drives turbines, which spin an electric generator. In contrast to a boiling water reactor (BWR), pressure in the primary coolant loop prevents the water from boiling within the reactor. All light-water reactors use ordinary water as both coolant and neutron moderator. Most use anywhere from two to four vertically mounted steam generators; VVER reactors use horizontal steam generators.

A boiling water reactor (BWR) is a type of light water nuclear reactor used for the generation of electrical power. It is a design different from a Soviet RBMK. It is the second most common type of electricity-generating nuclear reactor after the pressurized water reactor (PWR), which is also a type of light water nuclear reactor. The main difference between a BWR and PWR is that in a BWR, the reactor core heats water, which turns to steam and then drives a steam turbine. In a PWR, the reactor core heats water, which does not boil. This hot water then exchanges heat with a lower pressure system, which turns water into steam that drives the turbine. The BWR was developed by the Argonne National Laboratory and General Electric (GE) in the mid-1950s. The main present manufacturer is GE Hitachi Nuclear Energy, which specializes in the design and construction of this type of reactor.

The gridiron pendulum was a temperature-compensated clock pendulum invented by British clockmaker John Harrison around 1726 and later modified by John Ellicott. It was used in precision clocks. In ordinary clock pendulums, the pendulum rod expands and contracts with changes in temperature. The period of the pendulum's swing depends on its length, so a pendulum clock's rate varied with changes in ambient temperature, causing inaccurate timekeeping. The gridiron pendulum consists of alternating parallel rods of two metals with different thermal expansion coefficients, such as steel and brass. The rods are connected by a frame in such a way that their different thermal expansions compensate for each other, so that the overall length of the pendulum, and thus its period, stays constant with temperature.

John Tyndall FRS (; 2 August 1820 – 4 December 1893) was a prominent 19th-century Irish physicist. His scientific fame arose in the 1850s from his study of diamagnetism. Later he made discoveries in the realms of infrared radiation and the physical properties of air, proving the connection between atmospheric CO2 and what is now known as the greenhouse effect in 1859.

In the philosophy of thermal and statistical physics, the Brownian ratchet or Feynman–Smoluchowski ratchet is an apparent perpetual motion machine of the second kind, first analysed in 1912 as a thought experiment by Polish physicist Marian Smoluchowski. It was popularised by American Nobel laureate physicist Richard Feynman in a physics lecture at the California Institute of Technology on May 11, 1962, during his Messenger Lectures series The Character of Physical Law in Cornell University in 1964 and in his text The Feynman Lectures on Physics as an illustration of the laws of thermodynamics. The simple machine, consisting of a tiny paddle wheel and a ratchet, appears to be an example of a Maxwell's demon, able to extract mechanical work from random fluctuations (heat) in a system at thermal equilibrium, in violation of the second law of thermodynamics. Detailed analysis by Feynman and others showed why it cannot actually do this.

A bimetallic strip is used to convert a temperature change into mechanical displacement. The strip consists of two strips of different metals which expand at different rates as they are heated. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled.

Lampworking is a type of glasswork in which a torch or lamp is used to melt the glass. Once in a molten state, the glass is formed by blowing and shaping with tools and hand movements. It is also known as flameworking or torchworking, as the modern practice no longer uses oil-fueled lamps. Although lack of a precise definition for lampworking makes it difficult to determine when this technique was first developed, the earliest verifiable lampworked glass is probably a collection of beads thought to date to the fifth century BC. Lampworking became widely practiced in Murano, Italy in the 14th century. As early as the 17th century, itinerant glassworkers demonstrated lampworking to the public. In the mid-19th century lampwork technique was extended to the production of paperweights, primarily in France, where it became a popular art form, still collected today. Lampworking differs from glassblowing in that glassblowing uses a furnace as the primary heat source, although torches are also used.

Zirconium tungstate (ZrWO4) is the zirconium salt of tungstic acid and has unusual properties. The phase formed at ambient pressure by reaction of ZrO2 and WO3 is a metastable cubic phase, which has negative thermal expansion characteristics, namely it shrinks over a wide range of temperatures when heated. In contrast to most other ceramics exhibiting negative CTE (coefficient of thermal expansion), the CTE of ZrW2O8 is isotropic and has a large negative magnitude (average CTE of -7.2x10−6K−1) over a wide range of temperature (-273 °C to 777 °C). A number of other phases are formed at high pressures.

Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions.
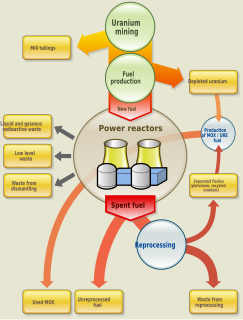
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.

The Chemical History of a Candle was the title of a series of six lectures on the chemistry and physics of flames given by Michael Faraday at the Royal Institution in 1848, as part of the series of Christmas lectures for young people founded by Faraday in 1825 and still given there every year.

This Timeline of heat engine technology describes how heat engines have been known since antiquity but have been made into increasingly useful devices since the 17th century as a better understanding of the processes involved was gained. A heat engine is any system that converts heat to mechanical energy, which can then be used to do mechanical work.They continue to be developed today.

Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices.
Thermophoresis is a phenomenon observed in mixtures of mobile particles where the different particle types exhibit different responses to the force of a temperature gradient. This phenomenon tends to move light molecules to hot regions and heavy molecules to cold regions. The term thermophoresis most often applies to aerosol mixtures whose mean free path is comparable to its characteristic length scale , but may also commonly refer to the phenomenon in all phases of matter. The term Soret effect normally applies to liquid mixtures, which behave according to different, less well-understood mechanisms than gaseous mixtures. Thermophoresis may not apply to thermomigration in solids, especially multi-phase alloys.

In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter.

Leslie's cube is a device used in the measurement or demonstration of the variations in thermal radiation emitted from different surfaces at the same temperature.
Copper–tungsten is a mixture of copper and tungsten. As copper and tungsten are not mutually soluble, the material is composed of distinct particles of one metal dispersed in a matrix of the other one. The microstructure is therefore rather a metal matrix composite instead of a true alloy.
The photoacoustic effect or optoacoustic effect is the formation of sound waves following light absorption in a material sample. In order to obtain this effect the light intensity must vary, either periodically or as a single flash. The photoacoustic effect is quantified by measuring the formed sound with appropriate detectors, such as microphones or piezoelectric sensors. The time variation of the electric output from these detectors is the photoacoustic signal. These measurements are useful to determine certain properties of the studied sample. For example, in photoacoustic spectroscopy, the photoacoustic signal is used to obtain the actual absorption of light in either opaque or transparent objects. It is useful for substances in extremely low concentrations, because very strong pulses of light from a laser can be used to increase sensitivity and very narrow wavelengths can be used for specificity. Furthermore, photoacoustic measurements serve as a valuable research tool in the study of the heat evolved in photochemical reactions, particularly in the study of photosynthesis.


















