Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

Ethyl carbamate (also called urethane) is an organic compound with the formula CH3CH2OC(O)NH2. It is an ester of carbamic acid and a white solid. Despite its name, it is not a component of polyurethanes. Because it is a carcinogen, it is rarely used, but naturally forms in low quantities in many types of fermented foods and drinks.

In organic chemistry, a carbamate is a category of organic compounds with the general formula R2NC(O)OR and structure >N−C(=O)−O−, which are formally derived from carbamic acid. The term includes organic compounds, formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H2NCOO−.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.

Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.
The molecular formula CH3NO2 may refer to:

Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of carbamic acid (H2NCO2H). It is a colourless solid.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is an acid-labile protecting group used in organic synthesis.

Chlorosulfonyl isocyanate is the chemical compound ClSO2NCO, known as CSI. This compound is a versatile reagent in organic synthesis.

Ethinamate is a short-acting carbamate-derivative sedative-hypnotic medication used to treat insomnia. Regular use leads to drug tolerance, and it is usually not effective for more than 7 days. Prolonged use can lead to dependence.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields ammonium carbamate [NH4]+[NH2CO2]−. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.

Carbamoyl phosphate synthetase catalyzes the ATP-dependent synthesis of carbamoyl phosphate from glutamine or ammonia and bicarbonate. This ATP-grasp enzyme catalyzes the reaction of ATP and bicarbonate to produce carboxy phosphate and ADP. Carboxy phosphate reacts with ammonia to give carbamic acid. In turn, carbamic acid reacts with a second ATP to give carbamoyl phosphate plus ADP.

Diphenylphosphoryl azide (DPPA) is an organic compound. It is widely used as a reagent in the synthesis of other organic compounds.

Benzyl carbamate is the organic compound with the formula C6H5CH2OC(O)NH2. The compound can be viewed as the ester of carbamic acid (O=C(OH)(NH2)) and benzyl alcohol, although it is produced from benzyl chloroformate with ammonia. It is a white solid that is soluble in organic solvents and moderately soluble in water. Benzyl carbamate is used as a protected form of ammonia in the synthesis of primary amines. After N-alkylation, C6H5CH2OC(O) group is removable with Lewis acids.

Pentabamate (S-109) is a tranquilizer of the carbamate family.
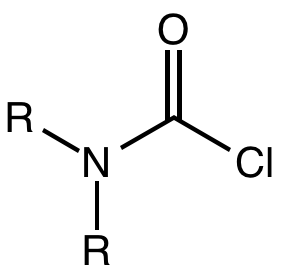
A carbamoyl chloride is the functional group with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb.

Ethiofencarb is a carbamate insecticide which is useful in controlling aphids on hard and soft fruits and some vegetables. It is not as dangerous as organophosphorous pesticides, but is considered highly toxic to humans in the UK, moderately toxic under US EPA classification, and highly toxic to aquatic life.

Chelidonine is an isolate of Papaveraceae with acetylcholinesterase and butyrylcholinesterase inhibitory activity.

Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions.

Allophanic acid is the organic compound with the formula H2NC(O)NHCO2H. It is a carbamic acid, the carboxylated derivative of urea. Biuret can be viewed as the amide of allophanic acid. The compound can be prepared by treating urea with sodium bicarbonate:
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.















