Related Research Articles

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

Michael James Steuart Dewar was an American theoretical chemist.

Frank Henry Westheimer was an American chemist. He taught at the University of Chicago from 1936 to 1954, and at Harvard University from 1953 to 1983, becoming the Morris Loeb Professor of Chemistry in 1960, and Professor Emeritus in 1983. The Westheimer medal was established in his honor in 2002.

Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in organic solvents. Uranocene, a member of the "actinocenes," a group of metallocenes incorporating elements from the actinide series. It is the most studied bis[8]annulene-metal system, although it has no known practical applications.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.

Group 2 organometallic chemistry refers to the organic derivativess of any group 2 element. It is a subtheme to main group organometallic chemistry. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are typically limited to academic interests.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
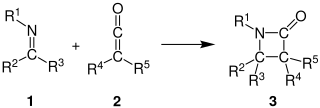
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.

Reductions with samarium(II) iodide involve the conversion of various classes of organic compounds into reduced products through the action of samarium(II) iodide, a mild one-electron reducing agent.
Janis Louie is a Chemistry professor and Henry Eyring Fellow at The University of Utah. Louie contributes to the chemistry world with her research in inorganic, organic, and polymer chemistry.

Transition metal isocyanide complexes are coordination compounds containing isocyanide ligands. Because isocyanides are relatively basic, but also good pi-acceptors, a wide range of complexes are known. Some isocyanide complexes are used in medical imaging.

Nitrogen pentahydride, also known as ammonium hydride is a hypothetical compound with the chemical formula NH5. There are two theoretical structures of nitrogen pentahydride. One structure is trigonal bipyramidal molecular geometry type NH5 molecule. Its nitrogen atom and hydrogen atoms are covalently bounded, and its symmetry group is D3h. Another predicted structure of nitrogen pentahydride is an ionic compound, composed of an ammonium ion and a hydride ion (NH4+H−). Until now, no one has synthesized this substance, or proved its existence, and related experiments have not directly observed nitrogen pentahydride. It is only speculated that it may be a reactive intermediate based on reaction products. Theoretical calculations show this molecule is thermodynamically unstable. The reason might be similar to the instability of nitrogen pentafluoride, so the possibility of its existence is low. However, nitrogen pentahydride might exist in special conditions or high pressure. Nitrogen pentahydride was considered for use as a solid rocket fuel for research in 1966.