
Ammonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct characteristic of a pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.

The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures:
Urea, also known as carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two –NH2 groups joined by a carbonyl (C=O) functional group.

Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients. Water bodies with very low nutrient levels are termed oligotrophic and those with moderate nutrient levels are termed mesotrophic. Advanced eutrophication may also be referred to as dystrophic and hypertrophic conditions.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Ichthyophthirius multifiliis, often termed "ICH", is a parasitic ciliate described by the French parasitologist Fouquet in 1876. Only one species is found in the genus which also gave name to the family. The name literally translates as "the fish louse with many children". The parasite can infect most freshwater fish species and, in contrast to many other parasites, shows very low host specificity. It penetrates gill epithelia, skin and fins of the fish host and resides as a feeding stage inside the epidermis. It is visible as a white spot on the surface of the fish but, due to its internal microhabitat, it is a true endoparasite and not an ectoparasite.
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests an alkali with composition [NH4+][OH−], it is actually impossible to isolate samples of NH4OH. The ions NH4+ and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions.

A reef aquarium or reef tank is a marine aquarium that prominently displays live corals and other marine invertebrates as well as fish that play a role in maintaining the tropical coral reef environment. A reef aquarium requires appropriately intense lighting, turbulent water movement, and more stable water chemistry than fish-only marine aquaria, and careful consideration is given to which reef animals are appropriate and compatible with each other.

A marine aquarium is an aquarium that keeps marine plants and animals in a contained environment. Marine aquaria are further subdivided by hobbyists into fish only (FO), fish only with live rock (FOWLR), and reef aquaria. Fish only tanks often showcase large or aggressive marine fish species and generally rely on mechanical and chemical filtration. FOWLR and reef tanks use live rock, a material composed of coral skeletons harboring beneficial nitrogen waste metabolizing bacteria, as a means of more natural biological filtration.
Community aquaria are tanks that are designed to contain more than one species of fish. Most commonly they include a variety of species that do not normally occur together in nature, for example angelfish from Brazil, swordtails from Mexico, and gouramis from South East Asia. The aim of such communities is to bring together fish that are compatible in temperament and water requirements, while using their different colours and behaviors to add interest and entertainment value.

Fishkeeping is a popular hobby, practiced by aquarists, concerned with keeping fish in a home aquarium or garden pond. There is also a piscicultural fishkeeping industry, as a branch of agriculture.

The common goldfish is a breed of goldfish with no other differences from its living ancestor, the Prussian carp, other than its color and shape. Goldfish are a form of domesticated wild carp and are a close relative of koi. Most varieties of fancy goldfish were derived from this simple breed. Common goldfish come in a variety of colors including red, orange, red/white, white/black, yellow/white, blue, grey/brown/, olive green, yellow, white, and black, with the most common variation being orange, hence the name. Sometimes, the brightness, duration, and the vividness of the color may be an indication of the fish's health status.
Ornamental fish kept in aquariums are susceptible to numerous diseases. Due to their generally small size and the low cost of replacing diseased or dead fish, the cost of testing and treating diseases is often seen as more trouble than the value of the fish.

Aquarium filters are critical components of both freshwater and marine aquaria. Aquarium filters remove physical and soluble chemical waste products from aquaria, simplifying maintenance. Furthermore, aquarium filters are necessary to support life as aquaria are relatively small, closed volumes of water compared to the natural environment of most fish.

An aquarium is a vivarium of any size having at least one transparent side in which aquatic plants or animals are kept and displayed. Fishkeepers use aquaria to keep fish, invertebrates, amphibians, aquatic reptiles, such as turtles, and aquatic plants. The term "aquarium", coined by English naturalist Philip Henry Gosse, combines the Latin root aqua, meaning water, with the suffix -arium, meaning "a place for relating to".
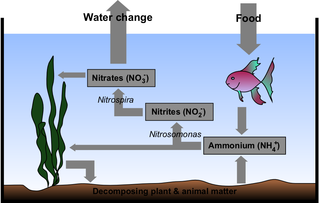
Fishless cycling is a form of "maturing" an aquarium. In this process, ammonia is provided to allow beneficial bacteria to colonize. Fishless cycling can reduce the chance of fish loss resulting from insufficient populations of these bacteria.
Ammoniacal nitrogen (NH3-N) is a measure for the amount of ammonia, a toxic pollutant often found in landfill leachate and in waste products, such as sewage, liquid manure and other liquid organic waste products. It can also be used as a measure of the health of water in natural bodies such as rivers or lakes, or in man made water reservoirs. The term is used widely in waste treatment and water purification systems.

An algae scrubber is a water filtering device which uses light to grow algae; in this process, undesirable chemicals are removed from the water. Algae scrubbers allow saltwater, freshwater and pond hobbyists to operate their tanks using natural filtration in the form of primary production, much like oceans and lakes.

Recirculating aquaculture systems (RAS) are used in home aquaria and for fish production where water exchange is limited and the use of biofiltration is required to reduce ammonia toxicity. Other types of filtration and environmental control are often also necessary to maintain clean water and provide a suitable habitat for fish. The main benefit of RAS is the ability to reduce the need for fresh, clean water while still maintaining a healthy environment for fish. To be operated economically commercial RAS must have high fish stocking densities, and many researchers are currently conducting studies to determine if RAS is a viable form of intensive aquaculture.

Ammonia pollution is pollution by the chemical ammonia (NH3) – a compound of nitrogen and hydrogen which is a byproduct of agriculture and industry. Common forms include air pollution by the ammonia gas emitted by rotting agricultural slurry and fertilizer factories while natural sources include the burning coal mines of Jharia, caustic Lake Natron and the guano of seabird colonies. Gaseous ammonia reacts with other pollutants in the air to form fine particles of ammonium salts which affect human breathing. Ammonia gas can also affect the soil chemistry of the locality that it settles on and will, for example, degrade the conditions required by the sphagnum moss and heathers of peatland.
















