
Malaria is a mosquito-borne infectious disease that affects humans and other vertebrates. Human malaria causes symptoms that typically include fever, fatigue, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin 10 to 15 days after being bitten by an infected Anopheles mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.

Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects. It can be taken by mouth or intravenously. Malaria resistance to quinine occurs in certain areas of the world. Quinine is also used as an ingredient in tonic water to impart a bitter taste.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.

Artemisia annua, also known as sweet wormwood, sweet annie, sweet sagewort, annual mugwort or annual wormwood, is a common type of wormwood native to temperate Asia, but naturalized in many countries including scattered parts of North America.
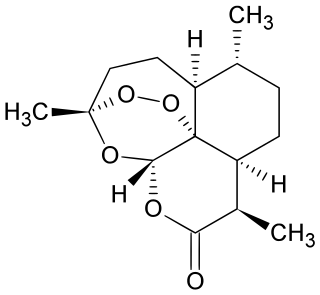
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Artemether is a medication used for the treatment of malaria. The injectable form is specifically used for severe malaria rather than quinine. In adults, it may not be as effective as artesunate. It is given by injection in a muscle. It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.

Artesunate (AS) is a medication used to treat malaria. The intravenous form is preferred to quinine for severe malaria. Often it is used as part of combination therapy, such as artesunate plus mefloquine. It is not used for the prevention of malaria. Artesunate can be given by injection into a vein, injection into a muscle, by mouth, and by rectum.

Plasmodium knowlesi is a parasite that causes malaria in humans and other primates. It is found throughout Southeast Asia, and is the most common cause of human malaria in Malaysia. Like other Plasmodium species, P. knowlesi has a life cycle that requires infection of both a mosquito and a warm-blooded host. While the natural warm-blooded hosts of P. knowlesi are likely various Old World monkeys, humans can be infected by P. knowlesi if they are fed upon by infected mosquitoes. P. knowlesi is a eukaryote in the phylum Apicomplexa, genus Plasmodium, and subgenus Plasmodium. It is most closely related to the human parasite Plasmodium vivax as well as other Plasmodium species that infect non-human primates.

Dihydroartemisinin is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs. It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.
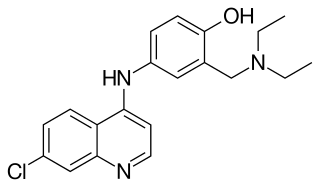
Amodiaquine (ADQ) is a medication used to treat malaria, including Plasmodium falciparum malaria when uncomplicated. It is recommended to be given with artesunate to reduce the risk of resistance. Due to the risk of rare but serious side effects, it is not generally recommended to prevent malaria. Though, the World Health Organization (WHO) in 2013 recommended use for seasonal preventive in children at high risk in combination with sulfadoxine and pyrimethamine.

Lumefantrine is an antimalarial drug. It is only used in combination with artemether. The term "co-artemether" is sometimes used to describe this combination. Lumefantrine has a much longer half-life compared to artemether, and is therefore thought to clear any residual parasites that remain after combination treatment.
Artesunate/amodiaquine, sold under the trade name Camoquin among others, is a medication used for the treatment of malaria. It is a fixed-dose combination of artesunate and amodiaquine. Specifically it recommended for acute uncomplicated Plasmodium falciparum malaria. It is taken by mouth.
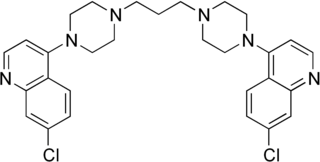
Piperaquine is an antiparasitic drug used in combination with dihydroartemisinin to treat malaria. Piperaquine was developed under the Chinese National Malaria Elimination Programme in the 1960s and was adopted throughout China as a replacement for the structurally similar antimalarial drug chloroquine. Due to widespread parasite resistance to piperaquine, the drug fell out of use as a monotherapy, and is instead used as a partner drug for artemisinin combination therapy. Piperaquine kills parasites by disrupting the detoxification of host heme.

Arterolane, also known as OZ277 or RBx 11160, is a substance that was tested for antimalarial activity by Ranbaxy Laboratories. It was discovered by US and European scientists who were coordinated by the Medicines for Malaria Venture (MMV). Its molecular structure is uncommon for pharmacological compounds in that it has both an ozonide (trioxolane) group and an adamantane substituent.
Project 523 is a code name for a 1967 secret military project of the People's Republic of China to find antimalarial medications. Named after the date the project launched, 23 May, it addressed malaria, an important threat in the Vietnam War. At the behest of Ho Chi Minh, Prime Minister of North Vietnam, Zhou Enlai, the Premier of the People's Republic of China, convinced Mao Zedong, Chairman of the Chinese Communist Party, to start the mass project "to keep [the] allies' troops combat-ready", as the meeting minutes put it. More than 500 Chinese scientists were recruited. The project was divided into three streams. The one for investigating traditional Chinese medicine discovered and led to the development of a class of new antimalarial drugs called artemisinins. Launched during and lasting throughout the Cultural Revolution, Project 523 was officially terminated in 1981.

Cipargamin is an experimental synthetic antimalarial drug belonging to the spiroindolone class. The compound was developed at the Novartis Institute for Tropical Diseases in Singapore, through a collaboration with the Genomics Institute of the Novartis Research Foundation (GNF), the Biomedical Primate Research Centre and the Swiss Tropical Institute.
Piperaquine/dihydroartemisinin (DHA/PPQ), sold under the brand name Eurartesim among others, is a fixed dose combination medication used in the treatment of malaria. It is a combination of piperaquine and dihydroartemisinin. Specifically it is used for malaria of the P. falciparum and P. vivax types. It is taken by mouth.
Zhou Yiqing is a professor of medicine at the Institute of Microbiology and Epidemiology of the People's Liberation Army Academy of Military Medical Sciences. He was one of the scientists who participated in the Project 523 of the Chinese Government under Chairman Mao Zedong. The project resulted in the discovery of artemisinins, a class of antimalarial drugs, from the medicinal plant Artemisia annua.

Ganaplacide is a drug in development by Novartis for the purpose of treating malaria. It belongs to the class of the imidazolopiperazines. It has shown activity against the Plasmodium falciparum and Plasmodium vivax forms of the malaria parasite.
Artesunate/pyronaridine, sold under the brand name Pyramax, is a fixed-dose combination medication for the treatment of malaria. It can be used for malaria of both the P. falciparum and P. vivax types. It combines artesunate and pyronaridine. It is taken by mouth.













