
Pressure measurement is the analysis of an applied force by a fluid on a surface. Pressure is typically measured in units of force per unit of surface area. Many techniques have been developed for the measurement of pressure and vacuum. Instruments used to measure and display pressure in an integral unit are called pressure gauges or vacuum gauges. A manometer is a good example, as it uses a column of liquid to both measure and indicate pressure. Likewise the widely used Bourdon gauge is a mechanical device, which both measures and indicates and is probably the best known type of gauge.
Standard conditions for temperature and pressure are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union of Pure and Applied Chemistry (IUPAC) and the National Institute of Standards and Technology (NIST), although these are not universally accepted standards. Other organizations have established a variety of alternative definitions for their standard reference conditions.
Relative density, or specific gravity, is the ratio of the density of a substance to the density of a given reference material. Specific gravity usually means relative density with respect to water. The term "relative density" is often preferred in scientific usage. It is defined as a ratio of density of particular substance with that of water.
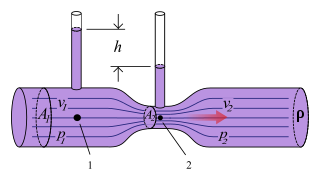
The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of a pipe. The Venturi effect is named after Giovanni Battista Venturi (1746–1822), an Italian physicist.
Permeability in fluid mechanics and the earth sciences is a measure of the ability of a porous material to allow fluids to pass through it.
A gas meter is a specialized flow meter, used to measure the volume of fuel gases such as natural gas and liquefied petroleum gas. Gas meters are used at residential, commercial, and industrial buildings that consume fuel gas supplied by a gas utility. Gases are more difficult to measure than liquids, because measured volumes are highly affected by temperature and pressure. Gas meters measure a defined volume, regardless of the pressurized quantity or quality of the gas flowing through the meter. Temperature, pressure, and heating value compensation must be made to measure actual amount and value of gas moving through a meter.

A mass flow meter, also known as an inertial flow meter is a device that measures mass flow rate of a fluid traveling through a tube. The mass flow rate is the mass of the fluid traveling past a fixed point per unit time.

A pressure sensor is a device for pressure measurement of gases or liquids. Pressure is an expression of the force required to stop a fluid from expanding, and is usually stated in terms of force per unit area. A pressure sensor usually acts as a transducer; it generates a signal as a function of the pressure imposed. For the purposes of this article, such a signal is electrical.

Hydraulic head or piezometric head is a specific measurement of liquid pressure above a vertical datum.

A mass (air) flow sensor (MAF) is a sensor used to determine the mass flow rate of air entering a fuel-injected internal combustion engine.
Standard cubic feet per minute (SCFM) is the molar flow rate of a gas corrected to "standardized" conditions of temperature and pressure thus representing a fixed number of moles of gas regardless of composition and actual flow conditions. It is related to the mass flow rate of the gas by a multiplicative constant which depends only on the molecular weight of the gas. There are different standard conditions for temperature and pressure, so care is taken when choosing a particular standard value. Worldwide, the "standard" condition for pressure is variously defined as an absolute pressure of 101,325 pascals, 1.0 bar, 14.73 psia, or 14.696 psia and the "standard" temperature is variously defined as 68 °F, 60 °F, 0 °C, 15 °C, 20 °C, or 25 °C. The relative humidity is also included in some definitions of standard conditions.
A wet gas is any gas with a small amount of liquid present. The term "wet gas" has been used to describe a range of conditions varying from a humid gas which is gas saturated with liquid vapour to a multiphase flow with a 90% volume of gas. There has been some debate as to its actual definition but there is currently no fully defined quantitative definition of a wet gas flow that is universally accepted.
Supercritical fluid chromatography (SFC) is a form of normal phase chromatography that uses a supercritical fluid such as carbon dioxide as the mobile phase. It is used for the analysis and purification of low to moderate molecular weight, thermally labile molecules and can also be used for the separation of chiral compounds. Principles are similar to those of high performance liquid chromatography (HPLC), however SFC typically utilizes carbon dioxide as the mobile phase; therefore the entire chromatographic flow path must be pressurized. Because the supercritical phase represents a state in which liquid and gas properties converge, supercritical fluid chromatography is sometimes called convergence chromatography.
In fluid measurement, the fluid's flow conditions refer to quantities like temperature and static pressure of the metered substance. The flowing conditions are required data in order to calculate the density of the fluid at flowing conditions. The flowing density is in turn required in order to compensate the measured volume to quantity at base conditions.

In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law.
Instrumentation is used to monitor and control the process plant in the oil, gas and petrochemical industries. Instrumentation comprises sensor elements, signal transmitters, controllers, indicators and alarms, actuated valves, logic circuits and operator interfaces.

A measuring instrument is a device for measuring a physical quantity. In the physical sciences, quality assurance, and engineering, measurement is the activity of obtaining and comparing physical quantities of real-world objects and events. Established standard objects and events are used as units, and the process of measurement gives a number relating the item under study and the referenced unit of measurement. Measuring instruments, and formal test methods which define the instrument's use, are the means by which these relations of numbers are obtained. All measuring instruments are subject to varying degrees of instrument error and measurement uncertainty.











