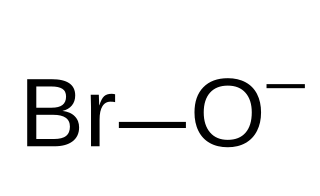Bromine is a chemical element with the symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process:
Sulfur oxide refers to many types of sulfur and oxygen containing compounds such as SO, SO2, SO3, S7O2, S6O2, S2O2, etc.

Chlorine and oxygen can bond in many ways:
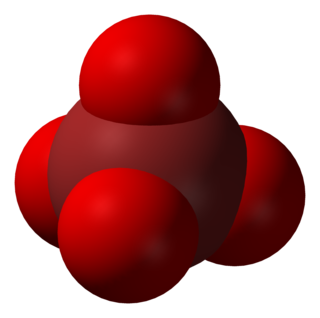
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.

Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.

Sodium bromate, the inorganic compound with the chemical formula of NaBrO3, is the sodium salt of bromic acid. It is a strong oxidant.
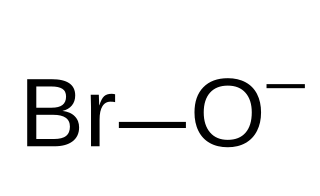
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. The Br–O bond length is 1.82 Å. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells. In many ways, hypobromite functions in the same manner as hypochlorite, and is also used as a germicide and antiparasitic in both industrial applications, and in the immune system.

Tantalum(V) bromide is the inorganic compound with the formula Ta2Br10. Its name comes from the compound's empirical formula, TaBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two TaBr5 units are joined by a pair of bromide bridges. There is no bond between the Ta centres. Niobium(V) chloride, niobium(V) bromide, niobium(V) iodide, tantalum(V) chloride, and tantalum(V) iodide all share this structural motif.
Antimony tribromide (SbBr3) is a chemical compound containing antimony in its +3 oxidation state.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Calcium bromide is the name for compounds with the chemical formula CaBr2(H2O)x. Individual compounds include the anhydrous material (x = 0), the hexahydrate (x = 6), and the rare dihydrate (x = 2). All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. The hydrated form is mainly used in some drilling fluids.

Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerization of chlorine dioxide (ClO2) at room temperature by 436 nm ultraviolet light:
Carbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound. It is a carbon oxohalide. Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.

Bismuth tribromide is an inorganic compound of bismuth and bromine with the chemical formula BiBr3.
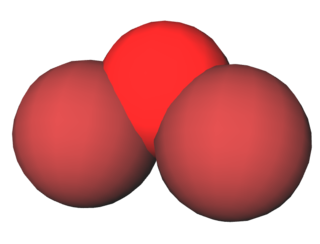
Dibromine monoxide is the chemical compound composed of bromine and oxygen with the formula Br2O. It is a dark brown solid which is stable below −40 °C and is used in bromination reactions. It is similar to dichlorine monoxide, the monoxide of its halogen neighbor one period higher on the periodic table. The molecule is bent, with C2v molecular symmetry. The Br−O bond length is 1.85 Å and the Br−O−Br bond angle is 112°, similar to dichlorine monoxide.

Dibromine trioxide is the chemical compound composed of bromine and oxygen with the formula Br2O3. It is an orange solid that is stable below −40 °C. It has the structure Br−O−BrO2 (bromine bromate). The Br−O−Br bond is bent, with a bond angle of 111.2°, and the Br−O−BrO2 bond length is 1.85Å.

Protactinium(V) fluoride is a fluoride of protactinium, with the chemical formula PaF5.

Bromine monoxide is a binary inorganic compound of bromine and oxygen with the chemical formula BrO. A free radical, this compound is the simplest of many bromine oxides. The compound is capable of influencing atmospheric chemical processes. Naturally, BrO can be found in volcanic plumes. BrO is similar to the oxygen monofluoride, chlorine monoxide and iodine monoxide radicals.