
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (—OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to a groups and a class of compounds with the same ring structure as the parent. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.
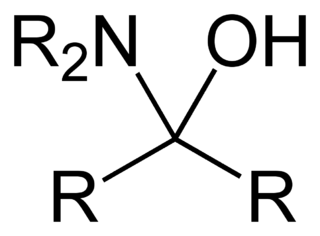
A hemiaminal (also carbinolamine) is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: -C(OH)(NR2)-. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution.

The bisulfite ion (IUPAC-recommended nomenclature: hydrogen sulfite) is the ion HSO3−. Salts containing the HSO3− ion are termed bisulfites also known as "sulfite lyes".
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonium chloride in the presence of potassium cyanide. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used commercially for the production of racemic methionine from methional.
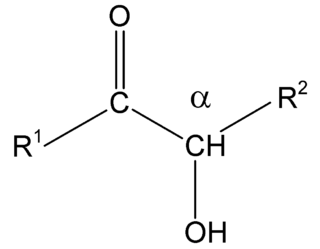
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The named acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups.
The Bucherer reaction in organic chemistry is the reversible conversion of a naphthol to a naphthylamine in the presence of ammonia and sodium bisulfite. The reaction is widely used in the synthesis of dye precursors aminonaphthalenesulfonic acids.
The Wulff–Dötz reaction (also known as the Dötz reaction or the benzannulation reaction of the Fischer carbene complexes) is the chemical reaction of an aromatic or vinylic alkoxy pentacarbonyl chromium carbene complex with an alkyne and carbon monoxide to give a Cr(CO)3-coordinated substituted phenol. Several reviews have been published. It is named after the German chemist Karl Heinz Dötz (b. 1943) and the American chemist William D. Wulff (b. 1949) at Michigan State University. The reaction was first discovered by Karl Dötz and was extensively developed by his group and W. Wulff's group. They subsequently share the name of the reaction.
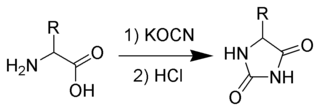
The Urech hydantoin synthesis is the chemical reaction of amino acids with potassium cyanate and hydrochloric acid to give hydantoins.

The Kaufmann–Bucherer–Neumann experiments measured the dependence of the inertial mass of an object on its velocity. The historical importance of this series of experiments performed by various physicists between 1901 and 1915 is due to the results being used to test the predictions of special relativity. The developing precision and data analysis of these experiments and the resulting influence on theoretical physics during those years is still a topic of active historical discussion, since the early experimental results at first contradicted Einstein's then newly published theory, but later versions of this experiment confirmed it. For modern experiments of that kind, see Tests of relativistic energy and momentum, for general information see Tests of special relativity.

Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole.
The Bucherer–Bergs reaction is the chemical reaction of carbonyl compounds or cyanohydrins with ammonium carbonate and potassium cyanide to give hydantoins. The reaction is named after Hans Theodor Bucherer.
Hans Theodor Bucherer was a German chemist and gave name to several chemical reactions, for example the Bucherer carbazole synthesis, the Bucherer reaction, and the Bucherer–Bergs reaction

Alfred Heinrich Bucherer was a German physicist, who is known for his experiments on relativistic mass. He also was the first who used the phrase "theory of relativity" for Einstein's theory of special relativity.

N-Vinylcarbazole is an organic compound used as a monomer in the production of poly(vinylcarbazole), a conductive polymer, in which conductivity is photon-dependent. The compound is used in the photoreceptors of photocopiers. Upon exposure to γ-irradiation, N-vinylcarbazole undergoes solid-state polymerisation.

The Borsche–Drechsel cyclization is a chemical reaction used to synthesize tetrahydrocarbazoles by the acid-catalyzed cyclization of cyclohexanone arylhydrazones. The reaction was first described by Edmund Drechsel in 1888 and by Walter Borsche in 1908.
A ring forming reaction or ring-closing reaction in organic chemistry is a general term for a variety of reactions that introduce one or more rings into a molecule. A heterocycle forming reaction is such a reaction that introduces a new heterocycle. Important classes of ring forming reactions include annulations and cycloadditions.
Aminonaphthalenesulfonic acids are compounds with the composition C10H6(NH2)(SO3H), being derived from naphthalene (C10H8) substituted by an amino and sulfonic acid groups. These compounds are colorless solids. They are useful precursors to dyes.

Tobias acid (2-amino-1-naphthalenesulfonic acid) is an organic compound with the formula C10H6(SO3H)(NH2). It is one of several aminonaphthalenesulfonic acids, which are derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear otherwise. It is used in the synthesis of azo dyes such as C.I. Acid Yellow 19 and C.I. Pigment Red 49. It is prepared via the Bucherer reaction of 2-hydroxynaphthalene-1-sulfonic acid with ammonia and ammonium sulfite.












