Related Research Articles
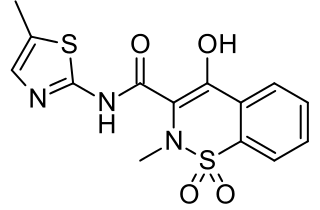
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory medication (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is used by mouth or by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

Bupivacaine, marketed under the brand name Marcaine among others, is a medication used to decrease feeling in a specific area. In nerve blocks, it is injected around a nerve that supplies the area, or into the spinal canal's epidural space. It is available mixed with a small amount of epinephrine to increase the duration of its action. It typically begins working within 15 minutes and lasts for 2 to 8 hours.
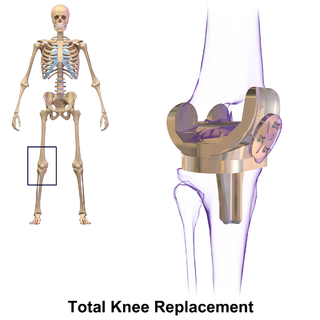
Knee replacement, also known as knee arthroplasty, is a surgical procedure to replace the weight-bearing surfaces of the knee joint to relieve pain and disability, most commonly offered when joint pain is not diminished by conservative sources and also for other knee diseases such as rheumatoid arthritis and psoriatic arthritis. In patients with severe deformity from advanced rheumatoid arthritis, trauma, or long-standing osteoarthritis, the surgery may be more complicated and carry higher risk. Osteoporosis does not typically cause knee pain, deformity, or inflammation and is not a reason to perform knee replacement.

Levobupivacaine (rINN) is a local anaesthetic drug indicated for minor and major surgical anaesthesia and pain management. It is a long-acting amide-type local anaesthetic that blocks nerve impulses by inhibiting sodium ion influx into the nerve cells. Levobupivacaine is the S-enantiomer of racemic bupivacaine and therefore similar in pharmacological effects. The drug typically starts taking effect within 15 minutes and can last up to 16 hours depending on factors such as site of administration and dosage.

Difluprednate, sold under the brand name Durezol, is a corticosteroid used for the treatment of post-operative ocular inflammation and pain.
Tanezumab is a monoclonal antibody against nerve growth factor as a treatment for pain via a novel mechanisms different from conventional pain-killer drugs. Tanezumab was discovered and developed by Rinat Neuroscience and was acquired by Pfizer in 2006.
Olaratumab, sold under the brand name Lartruvo, is a monoclonal antibody medication developed by Eli Lilly and Company for the treatment of solid tumors. It is directed against the platelet-derived growth factor receptor alpha.
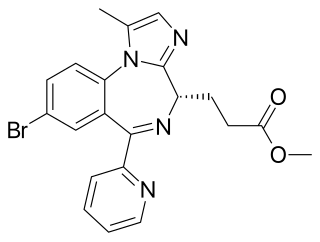
Remimazolam, sold under the brand name Byfavo, is a medication for the induction and maintenance of procedural sedation in adults for invasive diagnostic or surgical procedures lasting 30 minutes or less. It is a benzodiazepine drug, developed by PAION AG in collaboration with several regional licensees as an alternative to the short-acting imidazobenzodiazepine midazolam, for use in the induction of anesthesia and conscious sedation for minor invasive procedures. Remimazolam was found to have both a more rapid onset and a shorter duration than midazolam, and human clinical trials showed a faster recovery time and predictable, consistent pharmacokinetics, suggesting some advantages over existing drugs for these applications.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.

Difelikefalin, sold under the brand name Korsuva, is an analgesic opioid peptide used for the treatment of moderate to severe itching. It acts as a peripherally specific, highly selective agonist of the κ-opioid receptor (KOR).
Inebilizumab, sold under the brand name Uplizna, is a medication for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults. Inebilizumab is a humanized mAb that binds to and depletes CD19+ B cells including plasmablasts and plasma cells.
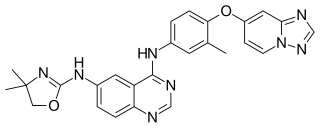
Tucatinib, sold under the brand name Tukysa, is an anticancer medication used for the treatment of HER2-positive breast cancer. It is a small molecule inhibitor of HER2. It was developed by Array BioPharma and licensed to Cascadian Therapeutics.
Umbralisib, sold under the brand name Ukoniq, is an anti-cancer medication for the treatment of marginal zone lymphoma (MZL) and follicular lymphoma (FL). It is taken by mouth.
Satralizumab, sold under the brand name Enspryng, is a humanized monoclonal antibody medication that is used for the treatment of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disease. The drug is being developed by Chugai Pharmaceutical, a subsidiary of Roche.
Bempedoic acid, sold under the brand name Nexletol among others, is a medication for the treatment of hypercholesterolemia.

Abrocitinib, sold under the brand name Cibinqo, is a medication used for the treatment of atopic dermatitis (eczema). It is a Janus kinase inhibitor and it was developed by Pfizer. It is taken by mouth.
A disease-modifying osteoarthritis drug (DMOAD) is a disease-modifying drug that would inhibit or even reverse the progression of osteoarthritis. Since the main hallmark of osteoarthritis is cartilage loss, a typical DMOAD would prevent the loss of cartilage and potentially regenerate it. Other DMOADs may attempt to help repair adjacent tissues by reducing inflammation. A successful DMOAD would be expected to show an improvement in patient pain and function with an improvement of the health of the joint tissues.
Trastuzumab/hyaluronidase, sold under the brand name Herceptin SC among others, is a fixed-dose combination medication for the treatment of HER2-overexpressing breast cancer in adults. It is a combination of trastuzumab and hyaluronidase.
Celecoxib/tramadol sold under the brand name Seglentis, is a fixed-dose combination of the anti-inflammatory celecoxib and the opioid tramadol used for the management and treatment of pain.
Cipaglucosidase alfa, sold under the brand name Pombiliti, and used in combination with miglustat, is a medication used for the treatment of glycogen storage disease type II. Cipaglucosidase alfa is a recombinant human acid α-glucosidase enzyme replacement therapy that provides an exogenous source of acid α-glucosidase.
References
- 1 2 3 4 5 6 "Zynrelef- bupivacaine and meloxicam solution". DailyMed. Retrieved 8 January 2022.
- 1 2 3 4 5 6 "Zynrelef EPAR". European Medicines Agency (EMA). 22 July 2020. Retrieved 6 October 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Zynrelef Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ↑ Blair HA (July 2021). "Bupivacaine/Meloxicam Prolonged Release: A Review in Postoperative Pain". Drugs. 81 (10): 1203–1211. doi:10.1007/s40265-021-01551-9. PMID 34228280. S2CID 235744803.
- 1 2 "Heron Therapeutics Announces U.S. FDA Approval of Zynrelef (HTX-011) for the Management of Postoperative Pain for up to 72 Hours" (Press release). Heron Therapeutics. 13 May 2021. Retrieved 14 May 2021– via PR Newswire.