
Arsenic is a chemical element with symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry.
Lead hydrogen arsenate, also called lead arsenate, acid lead arsenate or LA, chemical formula PbHAsO4, is an inorganic insecticide used primarily against the potato beetle.
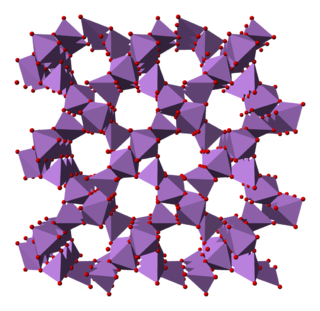
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All arsenic compounds are highly toxic and thus find only limited commercial applications.
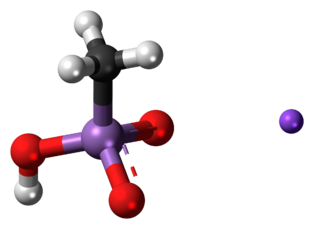
Monosodium methyl arsenate (MSMA) is an arsenic-based herbicide. It is an organo-arsenate; less toxic than the inorganic form of arsenates. However, the EPA states that all forms of arsenic are a serious risk to human health and the United States' Agency for Toxic Substances and Disease Registry ranked arsenic as number 1 in its 2001 Priority List of Hazardous Substances at Superfund sites.
Chromated copper arsenate (CCA) is a wood preservative that has been used for timber treatment since the mid-1930s. It is a mix of chromium, copper and arsenic formulated as oxides or salts, and is recognizable for the greenish tint it imparts to timber. CCA was invented in 1933 by Dr. Sonti Kamesam, an Indian scientist, and was awarded its first patent (British) in 1934.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless solid, it was originally used as a pesticide and as a germicide. It is highly soluble in water, as compared with lead arsenate, which makes it more toxic. The minerals Rauenthalite Ca3(AsO4)2·10H2O and Phaunouxite Ca3(AsO4)2·11H2O are hydrates of calcium arsenate.
Sodium arsenate is the inorganic compound with the formula Na3AsO4. Related salts are also called sodium arsenate, including Na2HAsO4 (disodium hydrogen arsenate) and NaH2AsO4 (sodium dihydrogen arsenate). The trisodium salt is a white or colourless solid that is highly toxic. It is usually handled as the dodecahydrate Na3AsO4.12H2O.
Arsenate reductase (donor) is an enzyme that catalyzes the chemical reaction
Arsenate reductase (glutaredoxin) (EC 1.20.4.1) is an enzyme that catalyzes the chemical reaction

Disodium methyl arsonate (DSMA) is the organoarsenic compound with the formula CH3AsO3Na2. It is a colorless, water-soluble solid derived from methanearsonic acid. It is used as a herbicide. Tradenames include Metharsinat, Arrhenal, Disomear, Metharsan, Stenosine, Tonarsan, Tonarsin, Arsinyl, Arsynal, and Diarsen.

Felisa Wolfe-Simon is an American microbial geobiologist and biogeochemist. In 2010, Wolfe-Simon led a team that discovered GFAJ-1, an extremophile bacterium that they claimed was capable of substituting arsenic for a small percentage of its phosphorus to sustain its growth, thus advancing the remarkable possibility of non-RNA/DNA-based genetics. However, these conclusions were immediately debated and criticized in correspondence to the original journal of publication, and have since come to be widely disbelieved. In 2012, two reports refuting the most significant aspects of the original results were published in the same journal in which the original findings had been previously published.

GFAJ-1 is a strain of rod-shaped bacteria in the family Halomonadaceae. It is an extremophile that was isolated from the hypersaline and alkaline Mono Lake in eastern California by geobiologist Felisa Wolfe-Simon, a NASA research fellow in residence at the US Geological Survey. In a 2010 Science journal publication, the authors claimed that the microbe, when starved of phosphorus, is capable of substituting arsenic for a small percentage of its phosphorus to sustain its growth. Immediately after publication, other microbiologists and biochemists expressed doubt about this claim which was robustly criticized in the scientific community. Subsequent independent studies published in 2012 found no detectable arsenate in the DNA of GFAJ-1, refuted the claim, and demonstrated that GFAJ-1 is simply an arsenate-resistant, phosphate-dependent organism.
Bacillus selenitireducens is a bacterium first isolated from Mono Lake, California. It is notable for respiring oxyanions of selenium and arsenic. It is spore-forming, rod-shaped and alkaliphile, its type strain being MLS10.
Bacillus arseniciselenatis is a bacterium first isolated from Mono Lake, California. It is notable for respiring oxyanions of selenium and arsenic. It is spore-forming, rod-shaped and alkaliphile, its type strain being E1H. It is a strict anaerobe.
Sulfurospirillum arsenophilum is a bacterium. It is notable for metabolising arsenic, hence its name.
Arsenate-reducing bacteria are bacteria which reduce arsenates. Arsenate-reducing bacteria are ubiquitous in arsenic-contaminated groundwater (aqueous environment). Arsenates are salts or esters of arsenic acid (H3AsO4), consisting of the ion AsO43−. They are moderate oxidizers that can be reduced to arsenites and to arsine. Arsenate can serve as a respiratory electron acceptor for oxidation of organic substrates and H2S or H2. Arsenates occur naturally in minerals such as adamite, alarsite, legrandite, and erythrite, and as hydrated or anhydrous arsenates. Arsenates are similar to phosphates since arsenic (As) and phosphorus (P) occur in group 15 (or VA) of the periodic table. Unlike phosphates, arsenates are not readily lost from minerals due to weathering. They are the predominant form of inorganic arsenic in aqueous aerobic environments. On the other hand, arsenite is more common in anaerobic environments, more mobile, and more toxic than arsenate. Arsenite is 25–60 times more toxic and more mobile than arsenate under most environmental conditions. Arsenate can lead to poisoning, since it can replace inorganic phosphate in the glyceraldehyde-3-phosphate --> 1,3-biphosphoglycerate step of glycolysis, producing 1-arseno-3-phosphoglycerate instead. Although glycolysis continues, 1 ATP molecule is lost. Thus, arsenate is toxic due to its ability to uncouple glycolysis. Arsenate can also inhibit pyruvate conversion into acetyl-CoA, thereby blocking the TCA cycle, resulting in additional loss of ATP.
Marinobacter santoriniensis is a Gram-negative, facultatively anaerobic, non-spore-forming and motile bacterium from the genus of Marinobacter which has been isolated from hydrothermal sediments in Santorini in Greece. Marinobacter santoriniensis can metabolize arsenate and arsenite.
Flavobacterium arsenatis is an arsenic-resistant, Gram-negative and rod-shaped bacterium from the genus of Flavobacterium which has been isolated from high-arsenic sediments from Jianghan Plain in China.
Tepidibacillus infernus is an aerotolerant anaerobic, organotrophic, spore-forming and moderately thermophilic bacterium from the genus of Tepidibacillus which has been isolated from microbial mat from the TauTona Gold Mine in South Africa.






