
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer (FRET), resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance.

A fluorescence microscope is an optical microscope that uses fluorescence and phosphorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a more simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image.

Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser confocal scanning microscopy (LCSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation. Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures within an object. This technique is used extensively in the scientific and industrial communities and typical applications are in life sciences, semiconductor inspection and materials science.
Fluorescence correlation spectroscopy (FCS) is a correlation analysis of fluctuation of the fluorescence intensity. The analysis provides parameters of the physics under the fluctuations. One of the interesting applications of this is an analysis of the concentration fluctuations of fluorescent particles (molecules) in solution. In this application, the fluorescence emitted from a very tiny space in solution containing a small number of fluorescent particles (molecules) is observed. The fluorescence intensity is fluctuating due to Brownian motion of the particles. In other words, the number of the particles in the sub-space defined by the optical system is randomly changing around the average number. The analysis gives the average number of fluorescent particles and average diffusion time, when the particle is passing through the space. Eventually, both the concentration and size of the particle (molecule) are determined. Both parameters are important in biochemical research, biophysics, and chemistry.

Stimulated emission depletion (STED) microscopy is one of the techniques that make up super-resolution microscopy. It creates super-resolution images by the selective deactivation of fluorophores, minimising the area of illumination at the focal point, and thus enhancing the achievable resolution for a given system. It was developed by Stefan W. Hell and Jan Wichmann in 1994, and was first experimentally demonstrated by Hell and Thomas Klar in 1999. Hell was awarded the Nobel Prize in Chemistry in 2014 for its development. In 1986, V.A. Okhonin had patented the STED idea. This patent was, perhaps, unknown to Hell and Wichmann in 1994.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.

Fluorescence cross-correlation spectroscopy (FCCS) was introduced by Eigen and Rigler in 1994 and experimentally realized by Schwille in 1997. It is essentially an extension of the fluorescence correlation spectroscopy (FCS) procedure by utilizing two differentially colored molecules, instead of one. In other words, coincident green and red intensity fluctuations of distinct molecules correlate if green and red labeled particles are moving together through a predefined confocal volume. As a result, FCCS provides a highly sensitive measurement of molecular interactions independent of diffusion rate. This is an important advancement, given that diffusion rate depends only weakly on the size of the molecular complex.

Nestor J. Zaluzec is an American scientist and inventor who works at Argonne National Laboratory. He invented and patented the Scanning Confocal Electron Microscope. and the π Steradian Transmission X-ray Detector for Electron-Optical Beam Lines and Microscopes.
Scanning confocal electron microscopy (SCEM) is an electron microscopy technique analogous to scanning confocal optical microscopy (SCOM). In this technique, the studied sample is illuminated by a focussed electron beam, as in other scanning microscopy techniques, such as scanning transmission electron microscopy or scanning electron microscopy. However, in SCEM, the collection optics is arranged symmetrically to the illumination optics to gather only the electrons that pass the beam focus. This results in superior depth resolution of the imaging. The technique is relatively new and is being actively developed.
The reduction-oxidation sensitive green fluorescent protein (roGFP) is a redox sensitive biosensor. Two cysteines were introduced into the beta barrel structure of the GFP. The oxidation state of the engineered thiols determines the fluorescence properties of the sensor. Originally, different roGFP versions were presented to allow the in vivo imaging of reducing compartments such as the cytosol (roGFP2). The cysteines introduced at the amino acid positions 147 and 204 produced the most robust results. roGFP2 preferentially interacts with glutaredoxins and therefore reports the cellular glutathione redox potential. The specificity of roGFP2 for glutathione is further increased by linking it to the human glutaredoxin 1 (Grx1). By expressing the Grx1-roGFP fusion sensors in the organism of interest and/or targeting the protein to a cellular compartment, it is possible to measure the glutathione redox potential in a specific cellular compartment in real-time and therefore provides major advantages compared to other invasive static methods e.g. HPLC.
Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules by means of fluorescence. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Super-resolution microscopy, in light microscopy, is a term that gathers several techniques, which allow images to be taken with a higher resolution than the one imposed by the diffraction limit. Due to the diffraction of light, the resolution in conventional light microscopy is limited, as stated by Ernst Abbe in 1873. In this context, a diffraction-limited microscope with numerical aperture N.A. and light with wavelength λ reaches a lateral resolution of d = λ/(2 N.A.) - a similar formalism can be followed for the axial resolution. The resolution for a standard optical microscope in the visible light spectrum is about 200 nm laterally and 600 nm axially. Experimentally, the attained resolution can be measured from the full width at half maximum (FWHM) of the point spread function (PSF) using images of point-like objects. Although the resolving power of a microscope is not well defined, it is generally considered that a super-resolution microscopy technique offers a resolution better than the one stipulated by Abbe.
The Virus Counter is an instrument for rapid quantification of viruses in liquid samples. It is a specialized flow cytometer that uses high-sensitivity fluorescence detection to give a direct measurement of the concentration of virus particles in a fraction of the time required for traditional plaque assays.
Photo-activated localization microscopy and stochastic optical reconstruction microscopy (STORM) are widefield fluorescence microscopy imaging methods that allow obtaining images with a resolution beyond the diffraction limit. The methods were proposed in 2006 in the wake of a general emergence of optical super-resolution microscopy methods, and were featured as Methods of the Year for 2008 by the Nature Methods journal. The development of PALM as a targeted biophysical imaging method was largely prompted by the discovery of new species and the engineering of mutants of fluorescent proteins displaying a controllable photochromism, such as photo-activatible GFP. However, the concomitant development of STORM, sharing the same fundamental principle, originally made use of paired cyanine dyes. One molecule of the pair, when excited near its absorption maximum, serves to reactivate the other molecule to the fluorescent state.
Endomicroscopy is a technique for obtaining histology-like images from inside the human body in real-time, a process known as ‘optical biopsy’. It generally refers to fluorescence confocal microscopy, although multi-photon microscopy and optical coherence tomography have also been adapted for endoscopic use. Commercially available clinical and pre-clinical endomicroscopes can achieve a resolution on the order of a micrometre, have a field-of-view of several hundred µm, and are compatible with fluorophores which are excitable using 488 nm laser light. The main clinical applications are currently in imaging of the tumour margins of the brain and gastro-intestinal tract, particularly for the diagnosis and characterisation of Barrett’s Esophagus, pancreatic cysts and colorectal lesions. A number of pre-clinical and transnational applications have been developed for endomicroscopy as it enables researchers to perform live animal imaging. Major pre-clinical applications are in gastro-intestinal tract, toumous margin detection, uterine complications, ischaemia, live imaging of cartilage and tendon, organoid imaging etc.
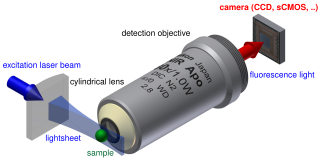
Light sheet fluorescence microscopy (LSFM) is a fluorescence microscopy technique with an intermediate-to-high optical resolution, but good optical sectioning capabilities and high speed. In contrast to epifluorescence microscopy only a thin slice of the sample is illuminated perpendicularly to the direction of observation. For illumination, a laser light-sheet is used, i.e. a laser beam which is focused only in one direction. A second method uses a circular beam scanned in one direction to create the lightsheet. As only the actually observed section is illuminated, this method reduces the photodamage and stress induced on a living sample. Also the good optical sectioning capability reduces the background signal and thus creates images with higher contrast, comparable to confocal microscopy. Because LSFM scans samples by using a plane of light instead of a point, it can acquire images at speeds 100 to 1000 times faster than those offered by point-scanning methods.

Live cell imaging is the study of living cells using time-lapse microscopy. It is used by scientists to obtain a better understanding of biological function through the study of cellular dynamics. Live cell imaging was pioneered in first decade of the 20th century. One of the first time-lapse microcinematographic films of cells ever made was made by Julius Ries, showing the fertilization and development of the sea urchin egg. Since then, several microscopy methods have been developed which allow researchers to study living cells in greater detail with less effort. A newer type of imaging utilizing quantum dots have been used as they are shown to be more stable. The development of holotomographic microscopy has disregarded phototoxicity and other staining-derived disadvantages by implementing digital staining based on cells’ refractive index.
Super-resolution dipole orientation mapping (SDOM) is a form of fluorescence polarization microscopy (FPM) that achieved super resolution through polarization demodulation. It was first described by Karl Zhanghao and others in 2016. Fluorescence polarization (FP) is related to the dipole orientation of chromophores, making fluorescence polarization microscopy possible to reveal structures and functions of tagged cellular organelles and biological macromolecules. In addition to fluorescence intensity, wavelength, and lifetime, the fourth dimension of fluorescence—polarization—can also provide intensity modulation without the restriction to specific fluorophores; its investigation in super-resolution microscopy is still in its infancy.











