Company overview
Cyclacel Pharmaceuticals, Inc. is a biopharmaceutical company developing oral therapies that target the various phases of cell cycle control for the treatment of cancer and other serious diseases. Sir David Lane, a recognized leader in the field of tumor suppressor biology, who discovered the p53 protein, founded the company in 1996. In 1999, Cyclacel Pharmaceuticals was joined by David Glover, a recognized leader in the mechanism of mitosis or cell division, who discovered, among other cell cycle targets, the mitotic kinases, Polo and Aurora, enzymes that act in the mitosis phase of the cell cycle.
Three product candidates are currently in clinical development. Sapacitabine (CYC682), a cell cycle modulating nucleoside analogue, failed its Phase 3 trial for the treatment of acute myeloid leukemia (AML) in the elderly. [1] Phase 1/2 studies are ongoing for the treatment of breast cancer and AML/MDS. Seliciclib (CYC202 or R-roscovitine), a CDK (cyclin dependent kinase) inhibitor, is in Phase 2 studies for the treatment of lung cancer and nasopharyngeal cancer and in a Phase 1 trial in combination with sapacitabine. CYC116, an Aurora kinase and VEGFR2 inhibitor, is in a Phase 1 trial in patients with solid tumors.
Cyclacel Pharmaceuticals' ALIGN Pharmaceuticals subsidiary markets directly in the U.S. Xclair Cream for radiation dermatitis, Numoisyn® Liquid and Numoisyn® Lozenges for xerostomia.
Reverse merger
After becoming the first European spin-out company to raise over $100 million in private equity, [2] the company had to pull out of a stock market listing in July 2004. [3] Cyclacel completed a reverse merger with Xcyte Therapies in December 2005 to acquire Nasdaq listing. [4] This allowed Cyclacel to raise an additional $45 million in April 2006 through a private placement of stock and warrants.

Celgene Corporation is a pharmaceutical company that makes cancer and immunology drugs. Its major product is Revlimid (lenalidomide), which is used in the treatment of multiple myeloma, and also in certain anemias. The company is incorporated in Delaware, headquartered in Summit, New Jersey, and a subsidiary of Bristol Myers Squibb (BMS).

Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.

Seliciclib is an experimental drug candidate in the family of pharmacological cyclin-dependent kinase (CDK) inhibitors that preferentially inhibit multiple enzyme targets including CDK2, CDK7 and CDK9, which alter the growth phase or state within the cell cycle of treated cells. Seliciclib is being developed by Cyclacel. This is a phase II, dose ranging, multicenter, randomized, double-blind, placebo-controlled study.

Aurora kinase A also known as serine/threonine-protein kinase 6 is an enzyme that in humans is encoded by the AURKA gene.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania

Aurora kinase inhibitors are a putative drug class for treating cancer. The Aurora kinase enzymes could be potential targets for novel small-molecule enzyme inhibitors.

Checkpoint kinase 1, commonly referred to as Chk1, is a serine/threonine-specific protein kinase that, in humans, is encoded by the CHEK1 gene. Chk1 coordinates the DNA damage response (DDR) and cell cycle checkpoint response. Activation of Chk1 results in the initiation of cell cycle checkpoints, cell cycle arrest, DNA repair and cell death to prevent damaged cells from progressing through the cell cycle.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Lestaurtinib is a tyrosine kinase inhibitor structurally related to staurosporine. This semisynthetic derivative of the indolocarbazole K252a was investigated by Cephalon as a treatment for various types of cancer. It is an inhibitor of the kinases fms-like tyrosine kinase 3 (FLT3), Janus kinase 2 (JAK2), tropomyosin receptor kinase (trk) A (TrkA), TrkB and TrkC.
CancerVax was an American pharmaceutical company founded in 1998 by Donald Morton. The company sought to develop a vaccine for cancer, and had candidates for melanoma reach phase III clinical trials. When those trials proved unsuccessful in 2005, the company soon underwent a reverse takeover with Micromet.

Sapacitabine is a chemotherapeutic drug developed by US biotechnology firm Cyclacel currently undergoing clinical trials against leukemia.

ARIAD Pharmaceuticals, Inc. was an American oncology company, now part of Takeda Oncology, which was founded in 1991 by Harvey J. Berger, M.D. and headquartered in Cambridge, Massachusetts. ARIAD engaged in the discovery, development, and commercialization of medicines for cancer patients.
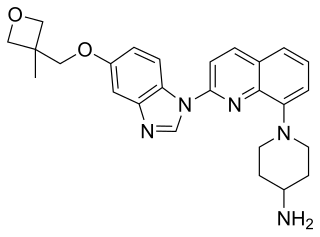
Crenolanib besylate is an investigational inhibitor being developed by AROG Pharmaceuticals, LLC. The compound is currently being evaluated for safety and efficacy in clinical trials for various types of cancer, including acute myeloid leukemia (AML), gastrointestinal stromal tumor (GIST), and glioma. Crenolanib is an orally bioavailable benzimidazole that selectively and potently inhibits signaling of wild-type and mutant isoforms of class III receptor tyrosine kinases (RTK) FLT3, PDGFR α, and PDGFR β. Unlike most RTK inhibitors, crenolanib is a type I mutant-specific inhibitor that preferentially binds to phosphorylated active kinases with the ‘DFG in’ conformation motif.

Volasertib is an experimental small molecule inhibitor of the PLK1 protein being developed by Boehringer Ingelheim for use as an anti-cancer agent. Volasertib is the second in a novel class of drugs called dihydropteridinone derivatives.

BI 811283 is a small molecule inhibitor of the Aurora B kinase protein being developed by Boehringer Ingelheim for use as an anti-cancer agent. BI 811283 is currently in the early stages of clinical development and is undergoing first in human trials in patients with solid tumors and acute myeloid leukemia.
Girentuximab is a chimeric IgG1 monoclonal antibody to carbonic anhydrase IX (CAIX). CAIX is expressed on the surface of most renal cancer cells and is hypothesized to be on the surface of other tumor cells. It is investigational agent in clinical trials for renal cell carcinoma. Its development was suspended as a "naked" or unconjugated antibody during phase III trials due to efficacy.

Kadmon Corporation is a biopharmaceutical company based in New York City. It also has operations in Warrendale, PA and Brighton, MA. The company was founded in 2009 by Samuel D. Waksal, founder and former CEO of ImClone Systems, a company now fully merged into Eli Lilly. Waksal had served a federal prison sentence stemming from his fiduciary role as CEO in the 2001 ImClone stock trading case. When released in 2009 he was barred from serving as an officer for any publicly traded company but Kadmon was privately financed.

Alisertib (MLN8237) is an orally available, investigational, reversible, ATP-competitive, selective aurora A kinase inhibitor developed by Takeda. Inhibition of aurora A kinase A leads to disruption of mitotic spindle apparatus assembly, disruption of chromosome segregation, and inhibition of cell proliferation.

György Kéri was a Hungarian biochemist, professor and Doctor of Biological Sciences (D.Sc.). His major field of research was signal transduction therapy, and he participated in the development of novel drug discovery technologies and drug candidates that entered the clinical development process.
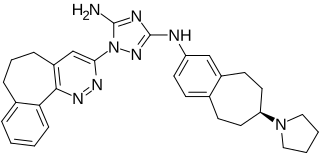
Bemcentinib, also known as BGB324 or R428, is an experimental oral small molecule that is an inhibitor of AXL kinase. Bemcentinib was licensed from Rigel Pharmaceuticals by BerGenBio and currently undergoing six Phase II trials in various solid and hematological tumors as monotherapy and in combination with immunotherapy, chemotherapy, and targeted therapeutics.


















