
Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs.

Mothballs are small balls of chemical pesticide and deodorant, sometimes used when storing clothing and other materials susceptible to damage from mold or moth larvae.

Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale. Phthalic acid is one of three isomers of benzenedicarboxylic acid, the others being isophthalic acid and terephthalic acid.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
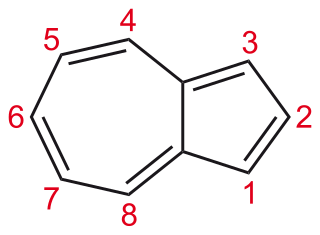
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.

1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.

Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

1,2-Naphthoquinone or ortho-naphthoquinone is a polycyclic aromatic organic compound with formula C
10H
6O
2. This yellow solid is prepared by oxidation of 1-amino-2-hydroxynaphthalene with ferric chloride.

Polychlorinated naphthalene (PCN) are the products obtained upon treatment of naphthalene with chlorine. The generic chemical formula is C10H8−(m+n)Cl(m+n). Commercial PCNs are mixtures of up to 75 components and byproducts. The material is an oil or a waxy solid, depending on the degree of chlorination. PCNs were once used in insulating coatings for electrical wires, as well as other applications, but their use has been largely phased out.

Naphthalenesulfonates are derivatives of sulfonic acid which contain a naphthalene functional unit. A related family of compounds are the aminonaphthalenesulfonic acids. Of commercial importance are the alkylnaphthalene sulfonates, which are used as superplasticizers in concrete. They are produced on a large scale by condensation of naphthalenesulfonate or alkylnaphthalenesulfonates with formaldehyde.

2-Naphthylamine is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.

Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. It is a partially hydrogenated derivative of naphthalene. It is a colorless liquid that is used as a hydrogen-donor solvent.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
Naftalan or Naphtalan is a type of crude oil. It is named after Naftalan, Azerbaijan, where it is found. It is known for its high naphthalene content and use in alternative medicine.
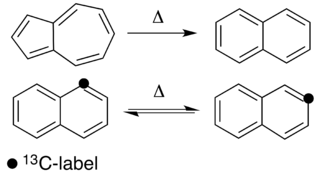
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.
Naphthalene poisoning is a form of poisoning that occurs when naphthalene is ingested. Severe poisoning can result in haemolytic anaemia. Naphthalene was introduced in 1841 by Rossbach as an antiseptic to counteract typhoid fever. Although naphthalene was widely used industrially, only nine cases of poisoning have been reported since 1947 as of 1956, suggesting underdiagnosis of the condition. As a result, the condition has limited coverage within medical journals.

Sodium naphthalene is an organic salt with the chemical formula Na+C
10H−
8. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When isolated, it invariably crystallizes as a solvate with ligands bound to Na+.

Lithium naphthalene is an organic salt with the chemical formula Li+C
10H−
8. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+.
















