
Dopamine is a hormone and a neurotransmitter that plays several important roles in the brain and body. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controlling the release of various hormones. These pathways and cell groups form a dopamine system which is neuromodulatory.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

Arvid Carlsson was a Swedish neuropharmacologist who is best known for his work with the neurotransmitter dopamine and its effects in Parkinson's disease. For his work on dopamine, Carlsson was awarded the Nobel Prize in Physiology or Medicine in 2000, together with Eric Kandel and Paul Greengard.
Dopa or DOPA may refer to:

l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is an amino acid that is made and used as part of the normal biology of humans, as well as some animals and plants. Humans, as well as a portion of the other animals that utilize l-DOPA in their biology, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme.
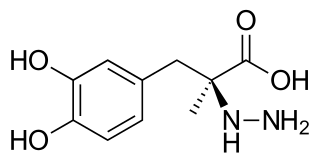
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of peripheral levodopa to cross the blood–brain barrier for central nervous system effect.
Dyskinesia refers to a category of movement disorders that are characterized by involuntary muscle movements, including movements similar to tics or chorea and diminished voluntary movements. Dyskinesia can be anything from a slight tremor of the hands to an uncontrollable movement of the upper body or lower extremities. Discoordination can also occur internally especially with the respiratory muscles and it often goes unrecognized. Dyskinesia is a symptom of several medical disorders that are distinguished by their underlying cause.
Carbidopa/levodopa, also known as levocarb and co-careldopa, is the combination of the two medications carbidopa and levodopa. It is primarily used to manage the symptoms of Parkinson's disease, but it does not slow down the disease or stop it from getting worse. It is taken by mouth. It can take two to three weeks of treatment before benefits are seen. Each dose then begins working in about ten minutes to two hours with a duration of effect of about five hours.

d-DOPA is similar to l-DOPA (levodopa), but with opposite chirality. Levo- and dextro- rotation refer to a molecule's ability to rotate planes of polarized light in either direction. Whereas l-DOPA is moderately effective in the treatment of Parkinson's disease (PD) and Dopamine-responsive dystonia (DRD) by stimulating the production of dopamine in the brain, d-DOPA is biologically inactive.

Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).
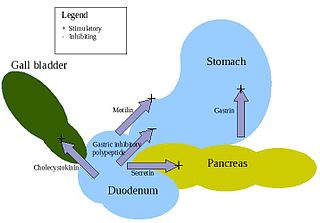
APUD cells constitute a group of apparently unrelated endocrine cells, which were named by the scientist A.G.E. Pearse, who developed the APUD concept in the early 1960s. These cells share the common function of secreting a low molecular weight polypeptide hormone. There are several different types which secrete the hormones secretin, cholecystokinin and several others. The name is derived from an acronym, referring to the following:
In enzymology, a dihydroxyphenylalanine ammonia-lyase (EC 4.3.1.11, entry deleted) is a non-existing enzyme that catalyzes the chemical reaction
In enzymology, a dihydroxyphenylalanine transaminase is an enzyme that catalyzes the chemical reaction

An aromatic L-amino acid decarboxylase inhibitor is a medication of type enzyme inhibitor which inhibits the synthesis of dopamine by the enzyme aromatic L-amino acid decarboxylase. It is used to inhibit the decarboxylation of L-DOPA to Dopamine outside the brain, i.e. in the blood. This is primarily co-administered with L-DOPA to combat Parkinson's disease. Administration can prevent common side-effects, such as nausea and vomiting, as a result of interaction with D2 receptors in the vomiting center located outside the blood–brain barrier.
3,4-dihydroxyphenylalanine oxidative deaminase (EC 1.13.12.15, 3,4-dihydroxy-L-phenylalanine: oxidative deaminase, oxidative deaminase, DOPA oxidative deaminase, DOPAODA) is an enzyme with systematic name 3,4-dihydroxy-L-phenylalanine:oxygen oxidoreductase (deaminating). This enzyme catalyses the following chemical reaction
3,4-dihydroxyphenylalanine reductive deaminase (EC 4.3.1.22, reductive deaminase, DOPA-reductive deaminase, DOPARDA) is an enzyme with systematic name 3,4-dihydroxy-L-phenylalanine ammonia-lyase (3,4-dihydroxyphenylpropanoate-forming). This enzyme catalyses the following chemical reaction

l-Dopaquinone also known as o-dopaquinone is a metabolite of L-DOPA (L-dihydroxyphenylalanine) and a precursor of melanin.
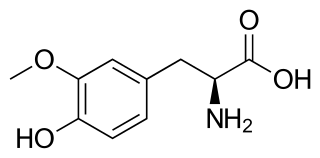
3-O-Methyldopa (3-OMD) is one of the most important metabolites of L-DOPA, a drug used in the treatment of the Parkinson's disease.
Jadwiga Bryła is a Polish biochemist. Since 1977 manager of Metabolism Regulation Department in Faculty of Biology in Warsaw University, and since 1983 professor in this faculty. Director of Biochemistry Institute. Since 1993 a member-correspondent of Polish Academy of Learning; she leads researches on regulations of intermediate transformations, especially on carbohydrates in animal tissues.
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.











