
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.

Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. It is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender people, and in hormonal birth control. It is also used in the treatment of prostate cancer. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.
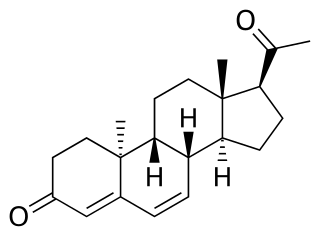
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Hydroxyprogesterone caproate, sold under the brand names Proluton and Makena among others, is a medication used to reduce the risk of preterm birth in women pregnant with one baby who have a history of spontaneous preterm birth. In March 2023, the manufacturer, Covis Pharma, agreed to withdraw the drug from the US market. The approvals of Makena and its generics were withdrawn by the US Food and Drug Administration (FDA) in April 2023.

Dienogest, sold under the brand name Visanne among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis. It is also used in menopausal hormone therapy and to treat heavy periods. Dienogest is available both alone and in combination with estrogens. It is taken by mouth.
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Algestone acetophenide, also known more commonly as dihydroxyprogesterone acetophenide (DHPA) and sold under the brand names Perlutal and Topasel among others, is a progestin medication which is used in combination with an estrogen as a form of long-lasting injectable birth control. It has also been used alone, but is no longer available as a standalone medication. DHPA is not active by mouth and is given once a month by injection into muscle.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.

Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications. It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations. OHPH is given by injection into muscle at regular intervals.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.
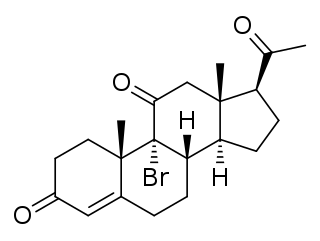
Bromoketoprogesterone (BKP), also known as 9α-bromo-11-oxoprogesterone (BOP), and known by the tentative brand name Braxarone (Squibb), is an orally active progestin which does not appear to have been marketed.

High-dose estrogen therapy (HDE) is a type of hormone therapy in which high doses of estrogens are given. When given in combination with a high dose of progestogen, it has been referred to as pseudopregnancy. It is called this because the estrogen and progestogen levels achieved are in the range of the very high levels of these hormones that occur during pregnancy. HDE and pseudopregnancy have been used in medicine for a number of hormone-dependent indications, such as breast cancer, prostate cancer, and endometriosis, among others. Both natural or bioidentical estrogens and synthetic estrogens have been used and both oral and parenteral routes may be used.

Estradiol benzoate/progesterone (EB/P4), sold under the brand names Duogynon and Sistocyclin among others, is a combination medication of estradiol benzoate (EB), an estrogen, and progesterone (P4), a progestogen. It has been formulated both as short-acting oil solutions and long-acting microcrystalline aqueous suspensions and is given by injection into muscle either once or continuously at regular intervals.

Estradiol valerate/hydroxyprogesterone caproate (EV/OHPC), sold under the brand names Gravibinon and Injectable No. 1 among others, is a combined estrogen and progestogen medication which is used in the treatment of threatened miscarriage and other indications and as a form of combined injectable birth control to prevent pregnancy. It contains estradiol valerate (EV), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin. The medication is given by injection into muscle once a day to once a month depending on the indication.

Estradiol benzoate/hydroxyprogesterone caproate (EB/OHPC), sold under the brand name Primosiston among others, is a combined estrogen and progestogen medication which is used to treat gynecological disorders and habitual abortion. It contains estradiol benzoate (EB), an estrogen, and hydroxyprogesterone caproate (OHPC), a progestin. The medication is given by injection into muscle.















