
Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disorders, and certain hormone-sensitive cancers. It is usually taken by mouth but is also used as a patch and vaginal ring.
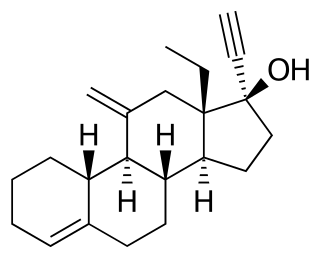
Desogestrel, sold under the brand names Cerazette and Mircette among many others, is a progestin medication which is used in birth control pills for women. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.

Drospirenone is a progestin medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is taken by mouth.
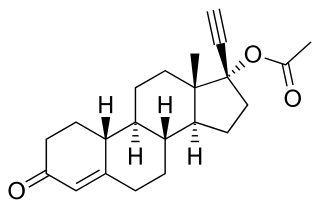
Norethisterone acetate (NETA), also known as norethindrone acetate and sold under the brand name Primolut-Nor among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication available in low-dose and high-dose formulations and is used alone or in combination with an estrogen. It is taken by mouth.

Norelgestromin, or norelgestromine, sold under the brand names Evra and Ortho Evra among others, is a progestin medication which is used as a method of birth control for women. The medication is available in combination with an estrogen and is not available alone. It is used as a patch that is applied to the skin.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Norgestrel, sold under the brand name Ovral among others, is a progestin medication which is used in birth control pills and in menopausal hormone therapy. It is available both in combination with an estrogen and alone. It is taken by mouth.
Ethinylestradiol/norethisterone acetate (EE/NETA), or ethinylestradiol/norethindrone acetate, is a combination of ethinylestradiol (EE) and norethisterone acetate (NETA) which is used as birth control. EE is an estrogen, while norethisterone acetate (NETA) is a progestin. It is taken by mouth. Some preparations of EE/NETA additionally contain an iron supplement in the form of ferrous fumarate.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.
Birth control pills come in a variety of formulations. The main division is between combined oral contraceptive pills, containing both estrogens and synthetic progestogens (progestins), and progestogen only pills. Combined oral contraceptive pills also come in varying types, including varying doses of estrogen, and whether the dose of estrogen or progestogen changes from week to week.
Ethinylestradiol/norethisterone (EE/NET), or ethinylestradiol/norethindrone, is a combination birth control pill which contains ethinylestradiol (EE), an estrogen and norethisterone (NET), a progestin. It is used for birth control, symptoms of menstruation, endometriosis, and menopausal symptoms. Other uses include acne. It is taken by mouth. Some preparations of EE/NET additionally contain an iron supplement in the form of ferrous fumarate.

Drospirenone/ethinylestradiol/levomefolic acid (EE/DRSP/LMF), sold under the brand names Beyaz among others, is a combination of ethinylestradiol (EE), an estrogen, drospirenone (DRSP), a progestogen, antimineralocorticoid, and antiandrogen, and levomefolic acid (LMF), a form of vitamin B9, which is used as a birth control pill to prevent pregnancy in women. The formulation contains folate as the calcium salt of levomefolic acid to lower the risk of complications such as fetal neural tube defects should the medication fail as a form of birth control. EE/DRSP/LMF was approved for use by the U.S. Food and Drug Administration (FDA) on 24 September 2010.

Ethinylestradiol/levonorgestrel (EE/LNG) is a combined birth control pill made up of ethinylestradiol, an estrogen and levonorgestrel a progestin. It is used for birth control, symptoms of menstruation, endometriosis, and as emergency contraception. It is taken by mouth. Some preparations of EE/LNG additionally contain an iron supplement in the form of ferrous bisglycinate or ferrous fumarate.

Ethinylestradiol sulfonate (EES), sold under the brand names Deposiston and Turisteron among others, is an estrogen medication which has been used in birth control pills for women and in the treatment of prostate cancer in men. It has also been investigated in the treatment of breast cancer in women. The medication was combined with norethisterone acetate in birth control pills. EES is taken by mouth once per week.
Combined birth control pills that contain natural estradiol or an estradiol ester include:

Ethinylestradiol/etonogestrel, sold under the brand names NuvaRing among others, is a hormonal vaginal ring used for birth control and to improve menstrual symptoms. It contains ethinylestradiol, an estrogen, and etonogestrel, a progestin. It is used by insertion into the vagina. Pregnancy occurs in about 0.3% of women with perfect use and 9% of women with typical use.
Conjugated estrogens/medroxyprogesterone acetate (CEs/MPA), sold under the brand names Prempro and Premphase, is a combination product of conjugated estrogens (Premarin), an estrogen, and medroxyprogesterone acetate (Provera), a progestogen, which is used in menopausal hormone therapy for the treatment of menopausal symptoms.

Ethinylestradiol/cyproterone acetate (EE/CPA), also known as co-cyprindiol and sold under the brand names Diane and Diane-35 among others, is a combination of ethinylestradiol (EE), an estrogen, and cyproterone acetate (CPA), a progestin and antiandrogen, which is used as a birth control pill to prevent pregnancy in women. It is also used to treat androgen-dependent conditions in women such as acne, seborrhea, excessive facial/body hair growth, scalp hair loss, and high androgen levels due to ovaries with cysts. The medication is taken by mouth once daily for 21 days, followed by a 7-day free interval.

Ethinylestradiol sulfonate/norethisterone acetate (EES/NETA), sold under the brand name Deposiston, is a combination medication of ethinylestradiol sulfonate (EES), an estrogen, and norethisterone acetate (NETA), a progestin, which was used as a combined birth control pill for women. It was formulated as oral tablets and contained 1 mg EES and 5 mg NETA per tablet. The medication had a long-lasting depot effect and was taken only once per week, for a total of four tablets per cycle. It was developed and marketed by Jenapharm and was previously available in Germany. EES/NETA was introduced for medical use in 1978.

Ethinylestradiol/drospirenone (EE/DRSP), sold under the brand name Yasmin among others, is a combination of ethinylestradiol (EE), an estrogen, and drospirenone (DRSP), a progestin, antimineralocorticoid, and antiandrogen, which is used as a birth control pill to prevent pregnancy in women. It is also indicated for the treatment of moderate acne, premenstrual syndrome (PMS), premenstrual dysphoric disorder (PMDD), and dysmenorrhea (painful menstruation) in women. The medication is taken by mouth and contains 30 μg EE and 3 mg DRSP per tablet (brand names Yasmin, others) or 20 μg EE and 3 mg DRSP per tablet (brand names Yaz, Yasminelle, Nikki, others). A formulation with levomefolic acid (vitamin B9) has also been marketed (brand names Beyaz, Safyral, others), with similar indications. EE/DRSP is marketed widely throughout the world.












