Related Research Articles

The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

Dopamine is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controlling the release of various hormones. These pathways and cell groups form a dopamine system which is neuromodulatory.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.
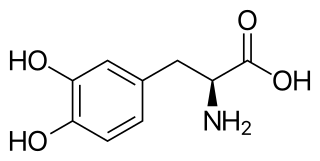
l-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize l-DOPA, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. In some plant families, l-DOPA is the central precursor of a biosynthetic pathway that produces a class of pigments called betalains. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.
Carbidopa (Lodosyn) is a drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of administered levodopa to cross the blood–brain barrier for central nervous system effect, instead of being peripherally metabolised into substances unable to cross said barrier.
The pars compacta (SNpc) is one of two subdivisions of the substantia nigra of the midbrain ; it is situated medial to the pars reticulata. It is formed by dopaminergic neurons. It projects to the striatum and portions of the cerebral cortex. It is functionally involved in fine motor control.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.
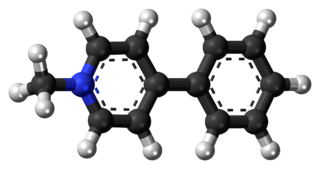
MPP+ (1-methyl-4-phenylpyridinium) is a positively charged organic molecule with the chemical formula C12H12N+. It is a neurotoxin that acts by interfering with oxidative phosphorylation in mitochondria by inhibiting complex I, leading to the depletion of ATP and eventual cell death.

Vacuolar protein sorting ortholog 35 (VPS35) is a protein involved in autophagy and is implicated in neurodegenerative diseases, such as Parkinson's disease (PD) and Alzheimer's disease (AD). VPS35 is part of a complex called the retromer, which is responsible for transporting select cargo proteins between vesicular structures and the Golgi apparatus. Mutations in the VPS35 gene (VPS35) cause aberrant autophagy, where cargo proteins fail to be transported and dysfunctional or unnecessary proteins fail to be degraded. There are numerous pathways affected by altered VPS35 levels and activity, which have clinical significance in neurodegeneration. There is therapeutic relevance for VPS35, as interventions aimed at correcting VPS35 function are in speculation.

Dopamine dysregulation syndrome (DDS) is a dysfunction of the reward system observed in some individuals taking dopaminergic medications for an extended length of time. It typically occurs in people with Parkinson's disease (PD) who have taken dopamine agonist medications for an extended period of time. It is characterized by problems such as addiction to medication, gambling, or sexual behavior.

Parkinson's disease (PD), or simply Parkinson's, is a long-term neurodegenerative disease of mainly the central nervous system that affects both the motor system and non-motor systems. The symptoms usually emerge slowly, and as the disease progresses, non-motor symptoms become more common. Usual symptoms are tremor, slowness of movement, rigidity, and difficulty with balance, collectively known as parkinsonism. Parkinson's disease dementia, falls and neuropsychiatric problems such as sleep abnormalities, psychosis, mood swings, or behavioral changes may arise in advanced stages.
Levodopa-induced dyskinesia (LID) is a form of dyskinesia associated with levodopa (l-DOPA), used to treat Parkinson's disease. It often involves hyperkinetic movements, including chorea, dystonia, and athetosis.
Parkinson's disease is the 2nd most prevalent neurological disorder within the United States and Europe, affecting around 1% of the population over the age of 60. While the link connecting the onset of Parkinson's disease to environmental factors is known, the link between dietary patterns and the disease is just beginning to be researched more fully. Additionally, other research has sought to examine the symptoms of the disease and propose methods on how to alleviate these symptoms through changes in diet. Current medications that work to alleviate the symptoms of Parkinson's disease can also be made more effective through changes in diet.
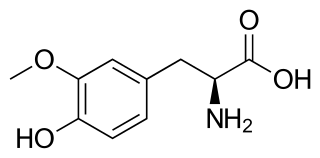
3-O-Methyldopa (3-OMD) is one of the most important metabolites of L-DOPA, a drug used in the treatment of the Parkinson's disease.

The pathophysiology of Parkinson's disease is death of dopaminergic neurons as a result of changes in biological activity in the brain with respect to Parkinson's disease (PD). There are several proposed mechanisms for neuronal death in PD; however, not all of them are well understood. Five proposed major mechanisms for neuronal death in Parkinson's Disease include protein aggregation in Lewy bodies, disruption of autophagy, changes in cell metabolism or mitochondrial function, neuroinflammation, and blood–brain barrier (BBB) breakdown resulting in vascular leakiness.

Animal models of Parkinson's disease are essential in the research field and widely used to study Parkinson's disease. Parkinson's disease is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of the dopamine neurons in the brain, results in motor dysfunction, ultimately causing the four cardinal symptoms of PD: tremor, rigidity, postural instability, and bradykinesia. It is the second most prevalent neurodegenerative disease, following Alzheimer's disease. It is estimated that nearly one million people could be living with PD in the United States.

Anders Björklund' is a Swedish neuroscientist and pioneer in the study of cell- and gene-based reparative and neuroprotective mechanisms in the brain. He has spent his academic career at Lund University in Sweden, as professor since 1983 and as senior professor at the Wallenberg Neuroscience Center since his formal retirement in 2012.

Epidemiological studies have shown lower age-related prevalence of Parkinson's disease in South Asians, with the rate of prevalence being around 52.7 per 100,000 as compared to a higher prevalence rate observed in populations with European origin, 108-257 per 100,000. Additionally, several studies have seen a higher prevalence of in women which contrasts with global data that observes a overall higher prevalence seen in men. Compared to most of the rest of the world, the South Asian countries seem to be on the lower end of PD prevalence. However, this is not to say that PD is not of concern in these countries. Over the past couple of years, the rate of Parkinson's has gone up in South Asia meaning that it is of high importance to study this pathological disease in these populations.
Parkinson's disease (PD), the second most common neurodegenerative disease after Alzheimer's disease, affects 1% of people over 60 years of age. In the past three decades, the number of PD cases has doubled globally from 2.5 million in 1990 to 6.1 million in 2016. As of 2022, there are ~10 million PD cases globally. In the United States, the estimated prevalence of PD by 2030 is estimated will be ~1.24 million. These numbers are expected to increase as life expectancy and the age of the general population increase. PD is considered to be a multisystem and multifactorial disease, where many factors, such as the environment, gut, lifestyle and genetics, play a significant role in the onset and progression of the disease.
References
- ↑ "Parkinson's disease: Diagnosis and management in primary and secondary care". National Institute for Health and Care Excellence. June 2006. Archived from the original on 26 January 2013. Retrieved 12 April 2013.
- 1 2 3 4 Feng, LR; Maguire-Zeiss, KA (March 2010). "Gene therapy in Parkinson's disease: rationale and current status". CNS Drugs. 24 (3): 177–92. doi:10.2165/11533740-000000000-00000. PMC 2886503 . PMID 20155994.
- 1 2 3 4 5 6 7 8 Coune, Philippe G; Schneider, Bernard L.; Aebischer, Patrick (April 2012). "Parkinson's Disease: Gene Therapies". Cold Spring Harbor Perspectives in Medicine. 2 (4): a009431. doi:10.1101/cshperspect.a009431. PMC 3312404 . PMID 22474617.
- 1 2 Horellou, Philippe; Mallet, Jacques (October 1997). "Ex Vivo Gene Therapy for Parkinson's Disease:lntracerebral Transplantation of Genetically Modified Cells". Molecular Neurobiology. 15 (2): 241–256. doi:10.1007/BF02740636. PMID 9396012. S2CID 34643353.
- ↑ Palfi, S. P.; Gurruchaga, J. M.; Ralph, G. S.; Lepetit, H.; Lavisse, S.; Buttery, P. C.; Watts, C.; Miskin, J.; Kelleher, M.; Deeley, S.; Iwamuro, H.; Lefaucheur, J. P.; Thiriez, C.; Fenelon, G.; Lucas, C.; Brugières, P.; Gabriel, I.; Abhay, K.; Drouot, X.; Tani, N.; Kas, A.; Ghaleh, B.; Le Corvoisier, P.; Dolphin, P.; Breen, D. P.; Mason, S.; Guzman, N. V.; Mazarakis, N. D.; Radcliffe, P. A.; Harrop, R. (2014). "Long-term safety and tolerability of Pro Savin, a lentiviral vector-based gene therapy for Parkinson's disease: A dose escalation, open-label, phase 1/2 trial". The Lancet. 383 (9923): 1138–1146. doi:10.1016/S0140-6736(13)61939-X. PMID 24412048. S2CID 4993549.
- 1 2 3 4 Forsayeth, J.; Bankiewicz, K. S.; Aminoff, M. J. (2010). "Gene therapy for Parkinson's disease: Where are we now and where are we going?". Expert Review of Neurotherapeutics. 10 (12): 1839–1845. doi:10.1586/ern.10.161. PMID 21091315. S2CID 19113338.
- ↑ Carlsson T, Winkler C, Burger C, Muzyczka N, Mandel RJ, Cenci A, Björklund A, Kirik D (March 2005). "Reversal of dyskinesias in an animal model of Parkinson's disease by continuous L-DOPA delivery using rAAV vectors". Brain. 128 (3): 559–69. doi: 10.1093/brain/awh374 . PMID 15659429.
- ↑ Campos-Romo, Aurelio; Ojeda-Flores, Rafael; Moreno-Briseño, Pablo; et al. (2012). "Behavioral improvement in MPtP-treated nonhuman primates in the HALLWAY task after transfer of tH cdnA to host astrocytes" (PDF). Acta Neurobiol. Exp. 72 (2): 166–176. doi:10.55782/ane-2012-1889. PMID 22810218. Archived from the original (PDF) on 23 September 2015. Retrieved 15 April 2013.
- ↑ "Gene Therapy Reverses Symptoms of Parkinson's Disease". Science Daily. Retrieved 12 April 2013.
- ↑ Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (April 1998). "Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism". Nature. 392 (6676): 605–8. Bibcode:1998Natur.392..605K. doi:10.1038/33416. PMID 9560156. S2CID 4432261.