Hydrion is a trademarked name for a popular line of compound pH indicators, marketed by Micro Essential Laboratory Inc., exhibiting a series of color changes (typically producing a recognizably different color for each pH unit) over a range of pH values. Although solutions are available, the most common forms of Hydrion are a series of papers impregnated with various mixtures of indicator dyes. It is considered a "universal indicator".

In chemistry, pH, also referred to as acidity, historically denotes "potential of hydrogen". It is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions are measured to have lower pH values than basic or alkaline solutions.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.

Søren Peter Lauritz Sørensen was a Danish chemist, famous for the introduction of the concept of pH, a scale for measuring acidity and alkalinity.
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C (standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring organic compounds are weak electrolytes, such as carboxylic acids and amines, pH indicators find many applications in biology and analytical chemistry. Moreover, pH indicators form one of the three main types of indicator compounds used in chemical analysis. For the quantitative analysis of metal cations, the use of complexometric indicators is preferred, whereas the third compound class, the redox indicators, are used in redox titrations (titrations involving one or more redox reactions as the basis of chemical analysis).

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.

Sputum is mucus that is coughed up from the lower airways. In medicine, sputum samples are usually used for a naked eye examination, microbiological investigation of respiratory infections and cytological investigations of respiratory systems. It is crucial that the specimen does not include any mucoid material from the nose or oral cavity.

Phenolphthalein ( feh-NOL(F)-thə-leen) is a chemical compound with the formula C20H14O4 and is often written as "HIn", "HPh", "phph" or simply "Ph" in shorthand notation. Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colorless in acidic solutions and pink in basic solutions. It belongs to the class of dyes known as phthalein dyes.
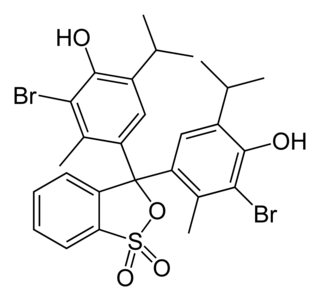
Bromothymol blue is a pH indicator. It is mostly used in applications that require measuring substances that would have a relatively neutral pH. A common use is for measuring the presence of carbonic acid in a liquid. It is typically sold in solid form as the sodium salt of the acid indicator.

Bromophenol blue, albutest is used as a pH indicator, an electrophoretic color marker, and a dye. It can be prepared by slowly adding excess bromine to a hot solution of phenolsulfonphthalein in glacial acetic acid.

Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the pKa of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity.

A universal indicator is a pH indicator made of a solution of several compounds that exhibit various smooth colour changes over a wide range pH values to indicate the acidity or alkalinity of solutions. Although there are several commercially available universal pH indicators, most are a variation of a formula patented by Yamada in 1933. Details of this patent can be found in Chemical Abstracts. Experiments with Yamada's universal indicator are also described in the Journal of Chemical Education.

Phenol red is a pH indicator frequently used in cell biology laboratories.
The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been mixed. For an acid-base reaction the equivalence point is where the moles of acid and the moles of base would neutralize each other according to the chemical reaction. This does not necessarily imply a 1:1 molar ratio of acid:base, merely that the ratio is the same as in the chemical reaction. It can be found by means of an indicator, for example phenolphthalein or methyl orange.

MacConkey agar is a selective and differential culture medium for bacteria. It is designed to selectively isolate Gram-negative and enteric bacteria and differentiate them based on lactose fermentation. Lactose fermenters turn red or pink on MacConkey agar, and nonfermenters do not change color. The media inhibits growth of Gram-positive organisms with crystal violet and bile salts, allowing for the selection and isolation of gram-negative bacteria. The media detects lactose fermentation by enteric bacteria with the pH indicator neutral red.
A complexometric indicator is an ionochromic dye that undergoes a definite color change in presence of specific metal ions. It forms a weak complex with the ions present in the solution, which has a significantly different color from the form existing outside the complex. Complexometric indicators are also known as pM indicators.
Consolidated Film Industries was a film laboratory and film processing company and was one of the leading film laboratories in the Los Angeles area for many decades. CFI processed negatives and made prints for motion pictures and television. The company and its employees received many Academy Awards for scientific or technical achievements.

Simmons' citrate agar is used for differentiating gram-negative bacteria on the basis of citrate utilization, especially for distinguishing Gammaproteobacteria of the family Enterobacteriaceae or even between species of the same genus. For example, Salmonella enteritidis would yield a positive (blue) result on Simmons’ agar and thus be distinguished from other Salmonella species like Salmonella typhi, Salmonella pullorum, and Salmonella gallinarum, which would yield a negative (green) result.

TacSat-2 is the first in a series of U.S. military experimental technology and communication satellites.TacSat-2 (also known as JWS-D1 was an experimental satellite built by the USAF's Air Force Research Laboratory with an operational life expected to be not more than one year as part of the "Advanced Concept Technology Demonstration" program.

The Motorola bag phone is the colloquial name for a line of personal transportable cellular telephones manufactured by Motorola, inc. from 1988 to 2000.
pHydrion is the trademarked name for a popular line of chemical test products, marketed by Micro Essential Laboratory, Inc., the original manufacturer of Hydrion and pHydrion products. The trademarked pHydrion product line comprises chemical test papers, chemical indicators, chemical test kits, chemical indicator kits, pH indicator pencils, chemical buffers, buffer salts, buffer preservatives, dispensers, color charts, and testing products, for use in testing, detecting, identifying, measuring, and indicating levels of pH, of sanitizers, and of other substances.