ISIS/Draw was a chemical structure drawing program developed by MDL Information Systems. It introduced a number of file formats for the storage of chemical information that have become industry standards. [1]

ISIS/Draw was a chemical structure drawing program developed by MDL Information Systems. It introduced a number of file formats for the storage of chemical information that have become industry standards. [1]
Molecular Design Limited (MDL) was founded by Stuart Marson and W. Todd Wipke in 1978, following the latter's experience with E. J. Corey in developing software for planning organic syntheses. [2] The company developed software to store and search chemical structures in large databases under the brand name MACCS (Molecular ACCess System), which was targeted at pharmaceutical and agrochemical companies. [3] [4] MDL offered their ISIS (Integrated Scientific Information System) products including ISIS/Draw as components of the MACCS system, specifically to allow chemists to use a graphical interface to register new compounds into corporate databases and to search these databases by structure or part-structure. They also introduced ISIS/Base, a chemical database program suited to the storage of relatively small numbers of structures with associated data for personal use independently of corporate systems. ISIS/Draw structures could be incorporated into other documents, for example using the word processor software which was becoming available in the 1980s, hence providing full electronic publishing for chemists. [5] MDL released many versions of the software and made ISIS/Draw freely available for non-commercial use: version 2.5 was available to run on Windows 98. [6] By 2007, MDL (then owned by Reed Elsevier) merged with Symyx Technologies, which in turn was acquired by Accelrys in 2010 and is now owned by Dassault Systèmes. The software is now branded as BIOVIA Draw.
MDL introduced specifications for chemical file formats, including the molfile (.mol), and structure/data file (.sdf) which were subsequently placed in the public domain and have become standards for representing structures in 2-D drawings and for transferring such information with associated data, for example identifiers, chemical names and substance properties. [1] [4] Many public databases implemented these standards and ChemSpider, for example, allows users to download molfiles for the structures it holds. [7] ISIS/Draw retained its own proprietary file formats with the extension .skc (sketch file) and .rxn (for reactions) and because of its role in preparing database queries it supported a variety of special atom and bond types used for substructure searching, such as wildcard atoms, aromatic bonds, ring bonds, and the atom mapping required for reaction searches.
While ISIS/Draw was mainly a 2D drawing program, it had some 3D rotation features and could interface with Rasmol for 3D visualization and rendering. ISIS/Draw also included structure and reaction validation features and could calculate elementary properties such as formula and molecular weight. It had an "AutoNom" add-in that allowed the creation of IUPAC names for valid structures and could use a special "pseudoatom" to generate amino-acid sequences for proteins. [6] [8]
One important feature of the MACCS system and ISIS/Draw was that it had comprehensive facilities, using hatched and wedged bonds, to represent relative or absolute stereochemistry and chirality and to recognise cis–trans isomerism in double bonds. In this respect it was superior to Wiswesser line notation [9] which had hitherto been used to create searchable databases. Likewise, while SMILES notation [10] can handle stereochemistry in some implementations it is more difficult for non-specialists to encode their structures in that way than by drawing them.
The current (2020) implementation of the software is called BIOVIA Draw and has several new features such as support for reading and writing International Chemical Identifiers (InChi) and converting IUPAC names into structure drawings. It is freely available for academic and non-commercial use. [11]
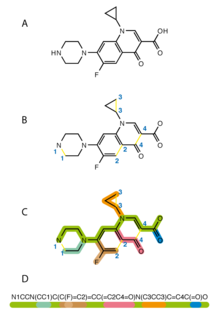
The simplified molecular-input line-entry system (SMILES) is a specification in the form of a line notation for describing the structure of chemical species using short ASCII strings. SMILES strings can be imported by most molecule editors for conversion back into two-dimensional drawings or three-dimensional models of the molecules.
Symyx Technologies, Inc. was a company that specialized in informatics and automation products. Symyx provided software solutions for scientific research, including Enterprise Laboratory Notebooks and products for combinatorial chemistry. The software part of the business became part of Accelrys, Inc. in 2010 and then in 2014 this company merged with Dassault Systèmes. Symyx also offered laboratory robotics systems for performing automated chemical research, which in 2010 was spun out as Freeslate, Inc.
A chemical database is a database specifically designed to store chemical information. This information is about chemical and crystal structures, spectra, reactions and syntheses, and thermophysical data.
A molecule editor is a computer program for creating and modifying representations of chemical structures.
This article discusses some common molecular file formats, including usage and converting between them.
Chemical table file is a family of text-based chemical file formats that describe molecules and chemical reactions. One format, for example, lists each atom in a molecule, the x-y-z coordinates of that atom, and the bonds among the atoms.
The IUPAC International Chemical Identifier is a textual identifier for chemical substances, designed to provide a standard way to encode molecular information and to facilitate the search for such information in databases and on the web. Initially developed by IUPAC and NIST from 2000 to 2005, the format and algorithms are non-proprietary.

JOELib is computer software, a chemical expert system used mainly to interconvert chemical file formats. Because of its strong relationship to informatics, this program belongs more to the category cheminformatics than to molecular modelling. It is available for Windows, Unix and other operating systems supporting the programming language Java. It is free and open-source software distributed under the GNU General Public License (GPL) 2.0.
MDL Information Systems, Inc. was a provider of R&D informatics products for the life sciences and chemicals industries. The company was launched as a computer-aided drug design firm in January 1978 in Hayward, California. The company was acquired by Symyx Technologies, Inc. in 2007. Subsequently Accelrys merged with Symyx. The Accelrys name was retained for the combined company. In 2014 Accelrys was acquired by Dassault Systemes. The Accelrys business unit was renamed BioVia.
ChemDraw is a molecule editor first developed in 1985 by David A. Evans and Stewart Rubenstein. The company was sold to PerkinElmer in the year 2011. ChemDraw, along with Chem3D and ChemFinder, is part of the ChemOffice suite of programs and is available for Macintosh and Microsoft Windows.
The SYBYL line notation or SLN is a specification for unambiguously describing the structure of chemical molecules using short ASCII strings. SLN differs from SMILES in several significant ways. SLN can specify molecules, molecular queries, and reactions in a single line notation whereas SMILES handles these through language extensions. SLN has support for relative stereochemistry, it can distinguish mixtures of enantiomers from pure molecules with pure but unresolved stereochemistry. In SMILES aromaticity is considered to be a property of both atoms and bonds whereas in SLN it is a property of bonds.
BIOVIA is a software company headquartered in the United States, with representation in Europe and Asia. It provides software for chemical, materials and bioscience research for the pharmaceutical, biotechnology, consumer packaged goods, aerospace, energy and chemical industries.
Kekulé was a computer program named after the chemist Friedrich August Kekulé von Stradonitz. The program was created starting in about 1990 by Joe McDaniel and Jason Balmuth while at Fein-Marquart Associates with funding from the National Cancer Institute under a Small Business Innovative Research Grant.
Wiswesser line notation (WLN), invented by William J. Wiswesser in 1949, was the first line notation capable of precisely describing complex molecules. It was the basis of ICI Ltd's CROSSBOW database system developed in the late 1960s. WLN allowed for indexing the Chemical Structure Index (CSI) at the Institute for Scientific Information (ISI). It was also the tool used to develop the CAOCI database, the datafile from which Accelrys' ACD file was developed. WLN is still being extensively used by BARK Information Services. Descriptions of how to encode molecules as WLN have been published in several books.
SMILES arbitrary target specification (SMARTS) is a language for specifying substructural patterns in molecules. The SMARTS line notation is expressive and allows extremely precise and transparent substructural specification and atom typing.

Chemical similarity refers to the similarity of chemical elements, molecules or chemical compounds with respect to either structural or functional qualities, i.e. the effect that the chemical compound has on reaction partners in inorganic or biological settings. Biological effects and thus also similarity of effects are usually quantified using the biological activity of a compound. In general terms, function can be related to the chemical activity of compounds.

Antony John Williams is a British chemist and expert in the fields of both nuclear magnetic resonance (NMR) spectroscopy and cheminformatics at the United States Environmental Protection Agency. He is the founder of the ChemSpider website that was purchased by the Royal Society of Chemistry in May 2009. He is a science blogger and an author.
ChemWindow is a chemical structure drawing molecule editor and publishing program now published by John Wiley & Sons in 2020, originally developed by Bio-Rad Laboratories, Inc. It was first developed by SoftShell International in the 1990s. Bio-Rad acquired this technology in 1996 and eventually made it part of their KnowItAll software product line, offering a specific ChemWindow edition of their software for structure drawing and publishing. They have also incorporated ChemWindow structure drawing components into their KnowItAll spectroscopy software packages with their DrawIt and ReportIt tools.
The hierarchical editing language for macromolecules (HELM) is a method of describing complex biological molecules. It is a notation that is machine readable to render the composition and structure of peptides, proteins, oligonucleotides, and related small molecule linkers.
The Modular Chemical Descriptor Language(MCDL) is a method for representing of molecular structures and pertinent molecular information using linear descriptors. MCDL files are designed for cross-platform transfer and manipulation of compound-specific chemical data. They consist of sets of unique information and nonunique information. The nonunique portion of the descriptor can be customized, thus providing end-user flexibility. Unique representation of atom and double bond stereochemistry is contrived as separate modules.