In thermodynamics, the specific heat capacity of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of 1 kg of water by 1 K is 4184 joules, so the specific heat capacity of water is 4184 J⋅kg−1⋅K−1.

Cumulus clouds are clouds which have flat bases and are often described as "puffy", "cotton-like" or "fluffy" in appearance. Their name derives from the Latin cumulo-, meaning heap or pile. Cumulus clouds are low-level clouds, generally less than 2,000 m (6,600 ft) in altitude unless they are the more vertical cumulus congestus form. Cumulus clouds may appear by themselves, in lines, or in clusters.

An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of anthropogenic aerosols include particulate air pollutants, mist from the discharge at hydroelectric dams, irrigation mist, perfume from atomizers, smoke, steam from a kettle, sprayed pesticides, and medical treatments for respiratory illnesses. When a person inhales the contents of a vape pen or e-cigarette, they are inhaling an anthropogenic aerosol.

Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects to float on a water surface without becoming even partly submerged.

The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elastic medium. At 20 °C (68 °F), the speed of sound in air is about 343 metres per second, or one kilometre in 2.9 s or one mile in 4.7 s. It depends strongly on temperature as well as the medium through which a sound wave is propagating. At 0 °C (32 °F), the speed of sound is about 331 m/s.

The lapse rate is the rate at which an atmospheric variable, normally temperature in Earth's atmosphere, falls with altitude. Lapse rate arises from the word lapse, in the sense of a gradual fall. In dry air, the adiabatic lapse rate is 9.8 °C/km.

Cloud physics is the study of the physical processes that lead to the formation, growth and precipitation of atmospheric clouds. These aerosols are found in the troposphere, stratosphere, and mesosphere, which collectively make up the greatest part of the homosphere. Clouds consist of microscopic droplets of liquid water, tiny crystals of ice, or both. Cloud droplets initially form by the condensation of water vapor onto condensation nuclei when the supersaturation of air exceeds a critical value according to Köhler theory. Cloud condensation nuclei are necessary for cloud droplets formation because of the Kelvin effect, which describes the change in saturation vapor pressure due to a curved surface. At small radii, the amount of supersaturation needed for condensation to occur is so large, that it does not happen naturally. Raoult's law describes how the vapor pressure is dependent on the amount of solute in a solution. At high concentrations, when the cloud droplets are small, the supersaturation required is smaller than without the presence of a nucleus.

In meteorology, convective available potential energy, is the integrated amount of work that the upward (positive) buoyancy force would perform on a given mass of air if it rose vertically through the entire atmosphere. Positive CAPE will cause the air parcel to rise, while negative CAPE will cause the air parcel to sink. Nonzero CAPE is an indicator of atmospheric instability in any given atmospheric sounding, a necessary condition for the development of cumulus and cumulonimbus clouds with attendant severe weather hazards.
The Wegener–Bergeron–Findeisen process, is a process of ice crystal growth that occurs in mixed phase clouds in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice. This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition. If the number density of ice is small compared to liquid water, the ice crystals can grow large enough to fall out of the cloud, melting into rain drops if lower level temperatures are warm enough.
A mass balance, also called a material balance, is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique. The exact conservation law used in the analysis of the system depends on the context of the problem, but all revolve around mass conservation, i.e., that matter cannot disappear or be created spontaneously.

The wet-bulb temperature (WBT) is the temperature read by a thermometer covered in water-soaked cloth over which air is passed. At 100% relative humidity, the wet-bulb temperature is equal to the air temperature ; at lower humidity the wet-bulb temperature is lower than dry-bulb temperature because of evaporative cooling.
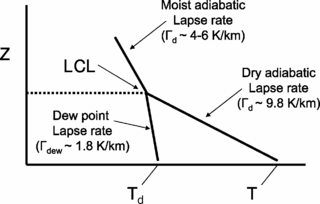
The lifted condensation level or lifting condensation level (LCL) is formally defined as the height at which the relative humidity (RH) of an air parcel will reach 100% with respect to liquid water when it is cooled by dry adiabatic lifting. The RH of air increases when it is cooled, since the amount of water vapor in the air remains constant, while the saturation vapor pressure decreases almost exponentially with decreasing temperature. If the air parcel is lifting further beyond the LCL, water vapor in the air parcel will begin condensing, forming cloud droplets. The LCL is a good approximation of the height of the cloud base which will be observed on days when air is lifted mechanically from the surface to the cloud base.
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is dependent upon thermodynamic principles and does not allude to special properties of materials. It is also used for determination of pore size distribution of a porous medium using adsorption porosimetry. The equation is named in honor of William Thomson, also known as Lord Kelvin.

The Twomey effect describes how additional cloud condensation nuclei (CCN), possibly from anthropogenic pollution, may increase the amount of solar radiation reflected by clouds. This is an indirect effect by such particles, as distinguished from direct effects (forcing) due to enhanced scattering or absorbing radiation by such particles not in clouds.

Köhler theory describes the process in which water vapor condenses and forms liquid cloud drops, and is based on equilibrium thermodynamics. It combines the Kelvin effect, which describes the change in saturation vapor pressure due to a curved surface, and Raoult's Law, which relates the saturation vapor pressure to the solute. It is an important process in the field of cloud physics. It was initially published in 1936 by Hilding Köhler, Professor of Meteorology in the Uppsala University.

Air pollutant concentrations, as measured or as calculated by air pollution dispersion modeling, must often be converted or corrected to be expressed as required by the regulations issued by various governmental agencies. Regulations that define and limit the concentration of pollutants in the ambient air or in gaseous emissions to the ambient air are issued by various national and state environmental protection and occupational health and safety agencies.
The rise in core (RIC) method is an alternate reservoir wettability characterization method described by S. Ghedan and C. H. Canbaz in 2014. The method enables estimation of all wetting regions such as strongly water wet, intermediate water, oil wet and strongly oil wet regions in relatively quick and accurate measurements in terms of Contact angle rather than wettability index.
The removal of heat from nuclear reactors is an essential step in the generation of energy from nuclear reactions. In nuclear engineering there are a number of empirical or semi-empirical relations used for quantifying the process of removing heat from a nuclear reactor core so that the reactor operates in the projected temperature interval that depends on the materials used in the construction of the reactor. The effectiveness of removal of heat from the reactor core depends on many factors, including the cooling agents used and the type of reactor. Common liquid coolants for nuclear reactors include: deionized water, heavy water, the lighter alkaline metals, lead or lead-based eutectic alloys like lead-bismuth, and NaK an eutectic alloy of sodium and potassium. Gas cooled reactors operate with coolants like carbon dioxide, helium or nitrogen but some very low powered research reactors have even been air-cooled with Chicago Pile 1 relying on natural convection of the surrounding air to remove the negligible thermal power output. There is ongoing research into using supercritical fluids as reactor coolants but thus far neither the supercritical water reactor nor a reactor cooled with supercritical Carbon Dioxide nor any other kind of supercritical-fluid-cooled reactor has ever been built.

This glossary of meteorology is a list of terms and concepts relevant to meteorology and atmospheric science, their sub-disciplines, and related fields.
The raindrop size distribution (DSD), or granulometry of rain, is the distribution of the number of raindrops according to their diameter (D). Three processes account for the formation of drops: water vapor condensation, accumulation of small drops on large drops and collisions between sizes. According to the time spent in the cloud, the vertical movement in it and the ambient temperature, the drops that have a very varied history and a distribution of diameters from a few micrometers to a few millimeters.
















