Neodymium fluoride may refer to:
- Neodymium(II) fluoride (Neodymium difluoride), NdF2
- Neodymium(III) fluoride (Neodymium trifluoride), NdF3
- Neodymium(IV) fluoride (Neodymium tetrafluoride), NdF4
Neodymium fluoride may refer to:

Neodymium is a chemical element; it has symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. It is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is widely distributed in the Earth's crust. Most of the world's commercial neodymium is mined in China, as is the case with many other rare-earth metals.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).
Neodymium chloride may refer to:
Bind or BIND may refer to:
Neodymium-doped yttrium lithium fluoride (Nd:YLF) is a lasing medium for arc lamp-pumped and diode-pumped solid-state lasers. The YLF crystal (LiYF4) is naturally birefringent, and commonly used laser transitions occur at 1047 nm and 1053 nm.
Yttrium lithium fluoride (LiYF4, sometimes abbreviated YLF) is a birefringent crystal, typically doped with neodymium or praseodymium and used as a gain medium in solid-state lasers. Yttrium is the substitutional element in LiYF4. The hardness of YLF is significantly lower than other commons crystalline laser media, i.e. yttrium aluminium garnet.
Trifluorides are compounds in which one atom or ion has three fluorine atoms or ions associated. Many metals form trifluorides, such as iron, the rare-earth elements, and the metals in the groups 3, 13 and 15 of the periodic table. Most metal trifluorides are poorly soluble in water except ferric fluoride and indium(III) fluoride, but several are soluble in other solvents.
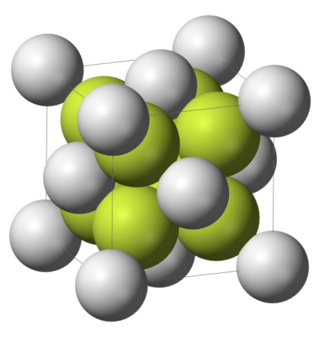
Difluorides are chemical compounds with two fluorine atoms per molecule.

Neodymium(III) bromide is an inorganic salt of bromine and neodymium the formula NdBr3. The anhydrous compound is an off-white to pale green solid at room temperature, with an orthorhombic PuBr3-type crystal structure. The material is hygroscopic and forms a hexahydrate in water (NdBr3· 6H2O), similar to the related neodymium(III) chloride.
Neodymium bromide may refer to:

Neodymium(III) fluoride is an inorganic chemical compound of neodymium and fluorine with the formula NdF3. It is a purplish pink colored solid with a high melting point.
Praseodymium(III) fluoride is an inorganic compound with the formula PrF3, being the most stable fluoride of praseodymium.
Neodymium(III) iodide is an inorganic salt of iodine and neodymium with the formula NdI3. Neodymium uses the +3 oxidation state in the compound. The anhydrous compound is a green powdery solid at room temperature.

Neodymium compounds are compounds formed by the lanthanide metal neodymium (Nd). In these compounds, neodymium generally exhibits the +3 oxidation state, such as NdCl3, Nd2(SO4)3 and Nd(CH3COO)3. Compounds with neodymium in the +2 oxidation state are also known, such as NdCl2 and NdI2. Some neodymium compounds have colors that vary based upon the type of lighting.
Neodymium iodide may refer to:

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
Neodymium hydride may refer to:

Neodymium(III) phosphate is an inorganic compound, with the chemical formula of NdPO4.
Neodymium(II) fluoride is an inorganic compound with the chemical formula NdF2. It can be obtained by shock compression of neodymium(III) fluoride and neodymium at 1000 °C and above 200 kbar. It can also be obtained by reacting in a eutectic system of neodymium and lithium fluoride-neodymium(III) fluoride at 1100 °C.